Polysorbates are a key type of nonionic, amphipathic surfactants that are utilized widely both clinically and preclinically in the pharmaceutical industry because of their relatively low toxicities and effectiveness at low concentrations (1).
They are utilized as surfactants to prevent surface absorption in the formulation of proteins, to limit physical damage during purification, filtration, freeze-drying, transportation, storage and delivery (3).
In the formulation of protein biopharmaceuticals, Polysorbate 80 (PS 80 – polyoxyethylene sorbitan monooleate) and Polysorbate 20 (PS 20 – polyoxyethylene sorbitan monolaurate) are the most common polysorbates utilized. They are made up of diverse mixtures of different fatty acid esters and the solutions which are sold by the manufacturers are labeled either as polysorbate 20 or 80 (Figure 1A).
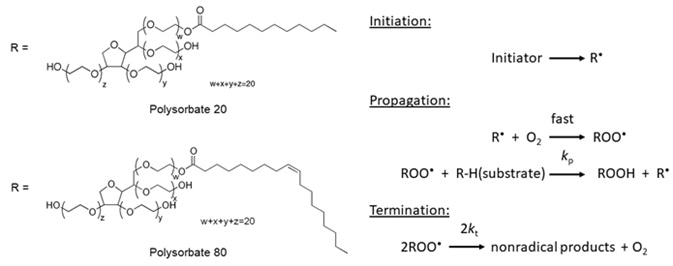
Figure 1A. Chemical structure of polysorbate 20 and polysorbate 80 (left). Figure 1B. General autoxidation mechanism (right).
It is well documented that polysorbates are prone to degradation by autoxidation. An example of a free radical chain reaction that results in polysorbate degradation is shown in Figure 1B. It begins with the oxidation of fatty acid esters, either by temperature-, metal-, or light-induced processes to form a number of fatty acid-free radicals. Typically, these free radicals are carbon-centered and quickly react with oxygen to form alkylperoxyl radicals.
By abstracting hydrogen atoms from other fatty acid ester molecules, alkylperoxyl radicals promote free radical formation further. The alkylperoxyl radicals are changed into hydroperoxides that either react with metals or undergo thermolysis to form alkoxyl, alkylperoxyl and carbon-centered free radicals. This free radical chain reaction carries on until it is stopped.
Stability testing under normal storage conditions is usually too slow for practical utilization in quality control, and these tests are frequently carried out under “forced” conditions as a result (e.g. using excessive exposure to light, elevated temperature, or in the presence of traces of transition metals) to start the degradation and the free radical process (4).
EPR (electron paramagnetic resonance, a.k.a ESR, electron spin resonance) is a spectroscopic method which directly and specifically measures samples which contain free radicals. At the same time the method is very specific, as it is totally “blind” to components within the samples that do not contain free radicals (or unpaired electrons).
It is required to add a compound known as a spin trap to enhance our capacity to detect free radicals as they are “shortlived.” The spin trap reacts with the free radical and forms a “radical adduct.” Radical adducts are also free radicals, but they are a lot more stable (their half-lives can be as long as days, compared to milliseconds).
One of the most favored spin traps is 5,5-dimethyl-1-pyrroline N-oxide (DMPO), which has been cited in Medline over 1,000 times. DMPO is redox inactive and the EPR spectra of the radical adducts are easily distinguishable, so it possesses huge benefits over other nitrone spin traps.
This article will explain how to use the EPR method to evaluate the level of degradation in polysorbates which have happened via autoxidation under normal laboratory storage (4 and 25 °C) or inappropriate transportation (40 °C) conditions, to give compelling evidence for the suitability of EPR as a quick technique for establishing the acceptable shelf-life of polysorbates.
Since the physiological temperature employed throughout testing and development of biological products is 37 °C, that is what was chosen as the forced degradation assay temperature.
Material and Methods
The spin trap DMPO was purchased from Dojindo Laboratories (Kumamoto, Japan). Glass capillaries and Critoseal were bought from VWR International. The polysorbates were obtained from three different vendors.
EPR spectra at every time point was gathered using a bench top EPR Bruker EMXnano spectrometer equipped with a variable temperature unit (VTU) with accessible temperature range of 100 - 425 K.
Using a 2D experiment (field sweep vs. time) configured in Bruker’s Xenon software, the formation of the spin trap radical adducts and their time evolution was observed. After the experimental data was gathered the radical concentration was established by utilizing double integration and the SpinCount module from the software.
Experimental Protocol
- Warm up the spin trap DMPO for 5-10 min to between 35 and 36 °C until it is liquid. The stock concentration is 9 M.
- Add 300 μl of polysorbate to an Eppendorf tube and then add 3 μl of the stock DMPO. The spin trap DMPO final concentration is around 100 mM.
- Vortex the tube and move the solution to a 100 μl capillary. Close the capillary with Critoseal.
- Put the capillary with the sample into the resonator and tune the spectrometer.
- Set the temperature to 37 °C using a variable temperature unit (VTU) and perform a 2D_Field_Delay experiment for 60 min.
Results
Effect of Temperature on the Radical Formation in Polysorbates
Two batches of polysorbates were stored for a month under two standard storage conditions (room temperature storage at 25 °C and refrigerated storage at 4 °C). A third batch was kept at a slightly elevated temperature of 40 °C for two weeks to speed up the degradation. By utilizing the experimental protocol outlined above, EPR spin trapping experiments were performed.
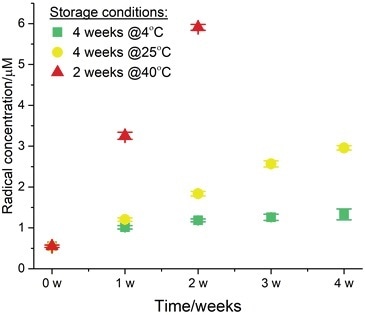
Figure 2.
For 60 minutes, at the physiological temperature of 37 °C, the formation of the radical adducts and their time evolution was monitored and the values at the 60 minute time points can be seen here. The EPR assay was then carried out once a week, for a total period of four weeks.
After the experimental data were gathered, by utilizing the SpinCount module from the Bruker Xenon software, quantitative EPR analysis was executed to calculate the radical concentration at the end point (t60).
Afterward, the data was exported and the t60 molarity was plotted versus storage time (Figure 2). This test exhibited that Tween® 20 contains 0.5 μM of free radicals as a baseline at t0. The sample which was stored for one month at 4 °C exhibited an increase by a factor of two, and the final radical concentration was 1 μM at the end-point t60 (show by the green squares).
The radical evolution gathered from the sample stored at room temperature (25 °C) for a month is shown by the yellow circles. The final concentration at the end of the experimental time period was approximately 3 μM which is an increase of a factor of more than 6 relative to t0.
The sample stored at 40 °C for two weeks had a radical yield of 6 μM which, compared to the baseline (red triangles), is more than a 30-fold increase. Overall, the radical yield corresponds directly with the level of degradation and shelf-life.
Generally, the results clearly show how EPR can be employed to establish the level of degradation and to forecast the long-term stability of polysorbates throughout typical storage and accelerated aging conditions.
Effect of Different Suppliers on the Radical Formation in Polysorbates
Polysorbates from three different suppliers were tested as received (i.e., at t0) under the same experimental conditions as described above with the objective of evaluating the relative oxidative status of the polysorbates received from the vendors (Figure 3).
In general, the degree of variation between the vendor and polysorbate type was surprisingly high. It exhibited a 7-fold variation which clearly indicates that during formulation development, careful selection and evaluation of product type and vendor is crucial. It is now possible to easily and routinely measure polysorbate samples from numerous vendors for QA/QC of incoming raw materials using EPR.
Conclusion
Polysorbates exist in a large amount of biopharmaceutical drugs and it is widely stated in the literature that caution must be shown when storing drug products that contain polysorbates.
The EPR data discussed in this article shows that degradation happens and is identified under a number of storage conditions leading to formation of free radicals, the main concern being that these radicals can readily oxidize and degrade proteins, resulting in undesired and potentially severe effects in patients.
There is surprising and significant differences between polysorbates bought from different vendors, showing that choice of both the vendor and polysorbate type are crucial. EPR spectroscopy is the only method for non-invasive and direct identification of free radicals in polysorbates. It is possible to identify, quantify and monitor the temporal behavior of the free radicals involved in the degradation of polysorbates by examining an EPR signal.
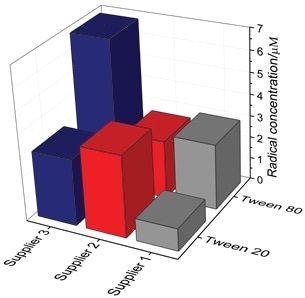
Figure 3. Radical concentration from three different suppliers. The molarity was determined at the end-point of the assay (t60).
References
- Ha E. et al., Peroxide formation in polysorbate 80 and protein stability, J. Pharm. Sci. (2002) 91 2252
- Harmon P. et al., A novel peroxy radical based oxidative stressing system for ranking the oxidizability of drug substances, J. Pharm. Sci. (2006) 95 2014
- Kerwin B. et al., Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: structure and degradation pathways, J. Pharm. Sci. (2002) 91 2252
- Ranguelova K. et al., Electron paramagnetic resonance: analysis of oxidative stability of olive oil, Inform (2013) 24 54
- Yao J. et al., A quantitative kinetic study of polysorbates autoxidation: the role of unsaturated fatty acid ester substituents, Pharm. Res. (2009) 26 2303
- Kishore R. et al., Degradation of polysorbates 20 and 80: studies of thermal autoxidation and hydrolysis, J. Pharm. Sci. (2011) 100 721
- Lam X. et al., Site-specific tryptophan oxidation induced by autocatalytic reaction of polysorbate 20 in protein formulation, Pharm. Res. (2011) 28 2543
- Maggio E. et al., Plysorbates, peroxides, protein aggregation, and immunogenicity – a growing concern, J. Excipients and Food Chem. (2012) 3 45
Appendix
Identification and quantification of detected free radicals in polysorbates is a key step in understanding the mechanism of their autoxidation. With the EPR quantification package (SpinCount and SpinFit) implemented in Bruker Xenon software, the task of identifying and quantifying the polysorbate radicals is both straightforward and precise.
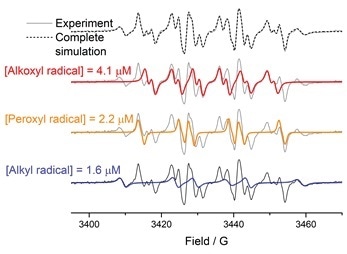
Figure A. An EPR spectrum of DMPO-radical adducts detected in polysorbates and the complete simulation are presented on the top in grey. From the simulated spectrum (the dotted trace) three different radicals were identified using SpinFit module – alkoxyl (simulated in red), peroxyl (simulated in orange), and alkyl (simulated in blue) radicals. The concentration of each radical species was determined using SpinCount module.
![The proposed mechanism of autoxidation in polysorbates strongly correlates with the EPR data. Reaction scheme was adapted from Ref. [3].](https://d2jx2rerrg6sh3.cloudfront.net/image-handler/picture/2019/5/5-2.jpg)
Figure B. The proposed mechanism of autoxidation in polysorbates strongly correlates with the EPR data. Reaction scheme was adapted from Ref. [3].
About Bruker BioSpin - NMR, EPR and Imaging

Bruker BioSpin offers the world's most comprehensive range of NMR and EPR spectroscopy and preclinical research tools. Bruker BioSpin develops, manufactures and supplies technology to research establishments, commercial enterprises and multi-national corporations across countless industries and fields of expertise.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.