Experiments have shown that surface coatings have an important role to play in cell movement.
Cell migration experiments will often involve considerations around which surface coatings should be employed in order to effectively culture a particular cell type.
Scientists working in the fields of wound healing, cancer research, and drug discovery will utilize cell cultures as part of vital experiments designed to advance the scientific community’s understanding of biological mechanisms.
Assays for cell migration facilitate the characterization of substances and conditions that influence cell movement; for example, scientists have successfully utilized the Oris™ Cell Migration Assay to identify proteins, antioxidants and mRNA with the ability to inhibit tumor cell migration.
Every cell type requires particular conditions for optimal growth. It is therefore essential to select the most appropriate surface to culture each cell type.
For example, fibroblasts produce and inhabit the body’s connective tissue, so it can be hypothesized that surfaces and scaffolds rich in collagen and other fibers (components of connective tissue) are more suitable for culturing fibroblasts.
Collagen-coated surfaces are a good option when culturing fibroblasts for cell migration assays, but it is also important to consider whether or not the choice of surface coating will also influence cell migration.
Scientists at Platypus Technologies have recently conducted a series of experiments to show that the choice of surface coating for a cell culture does have the potential to influence the results of cell migration experiments.
Oris™ Cell Migration Assays utilize a 96-well plate with special ‘stopper’ barriers designed to create a central cell-free detection zone for cell migration experiments. Removal of these stoppers will allow cells to migrate into the detection zone at the center of the well.
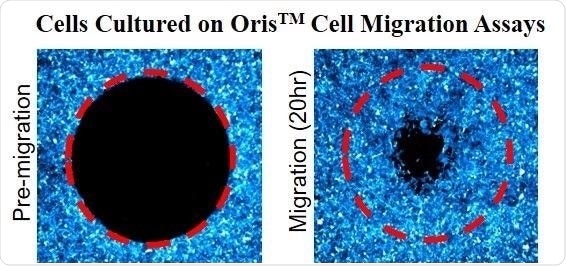
Principle of OrisTM Cell Migration Assay: OrisTM stoppers are used to create a central cell free Detection Zone (red dotted circle). Following 20 hours of incubation, the cells migrate into the detection zone. Image Credit: Platypus Technologies, LLC
Scientists conducting this experiment used an HT1080 cell line – fibroblasts that have been isolated from a malignant human tumor. These cells were incubated using Oris™ Cell Migration Assays.
The assays used wells coated with five different bioactive surfaces: Tissue Culture, Collagen I, Fibronectin, Poly-L-Lysine, and Basement Membrane Extract (BME).
After cell attachment was complete, the Oris™ stoppers were removed to allow cells to migrate into the detection zone. Cells were imaged after 24 hours of incubation without Oris™ stoppers. A range of representative images from this experiment is displayed below:
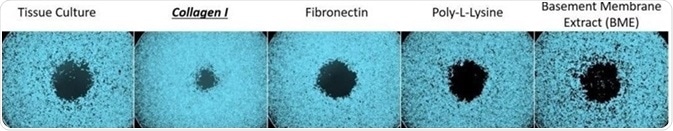
Representative images of cells (HT1080) cultured in surfaces treated with various bioactive coatings. OrisTM stoppers are used to create a central cell-free Detection Zone. Following 24 hours of incubation, the cells migrate into the detection zone. HT1080 cells cultured in Collagen I surfaces migrate faster than when cultured in other surfaces. Image Credit: Platypus Technologies, LLC
Visual inspection of these images indicated that cells cultured on collagen I were able to migrate deeper into the detection zone than cells that had been cultured on the other bioactive surfaces. There was no notable difference in the migration of the cells cultured on tissue culture, fibronectin, poly-l-lysine or BME.
Twelve different wells from each plate were imaged both before and after cell migration. ImageJ was used to measure the area of the cell-free zone, and the difference in the cell-free zone’s area pre- and post-migration was used to calculate the percentage area closure of the assay.
The graph below displays these measurements, which confirm that the HT1080 cells exhibit higher migration rates in Collagen I than in other surface coatings.
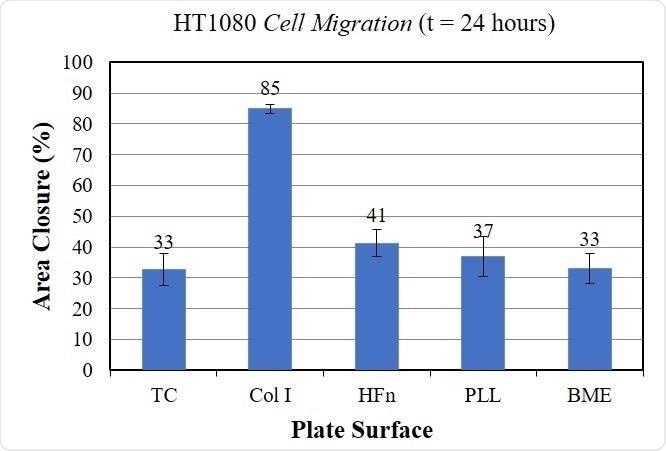
Quantitative Migration of Cells incubated on different bioactive surfaces. These data indicate that the HT1080 cells incubated on Collagen I (Col I) exhibit higher rates of cell migration. Image Credit: Platypus Technologies, LLC
The choice of surface coating was found to have a considerable impact on the cell migration of HT1080 cells. Cells cultured in collagen I exhibited the fastest rate of cell migration when compared to cells that had been cultured on other surfaces.
Overall, these results confidently demonstrate that the choice of surface coating has an important role to play in cell migration measurements.

This information has been sourced, reviewed and adapted from materials provided by Platypus Technologies, LLC.
For more information on this source, please visit Platypus Technologies, LLC.