Nuclear magnetic resonance (NMR) spectroscopy is a powerful research technique that exploits the magnetic characteristics of certain atomic nuclei. It is used to elucidate structure and understand molecular interactions and serves as a useful tool in environmental studies.
In this article, NMR is applied to natural water samples without any pre-treatment. Figure 1 shows the use of W5-Watergate water suppression, which suppresses the water signal below the spectrometer noise. Figure 2 shows the ocean, lake and river dissolved organic matter (DOM) at natural abundance. Although time consuming, the profiles of DOM at natural abundance supply a snap shot of the environment’s true state, making it possible to evaluate common isolation processes.
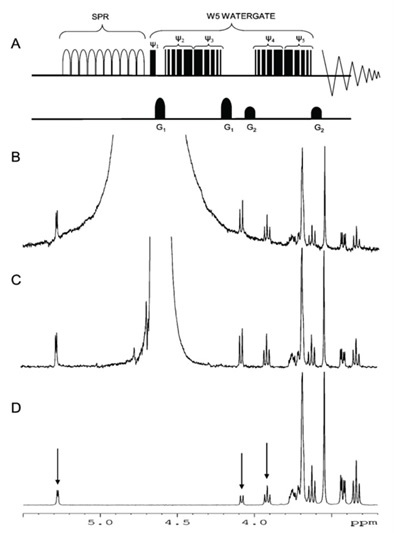
Figure 1. Watergate water suppression, suppressing the water signal below the spectrometer noise
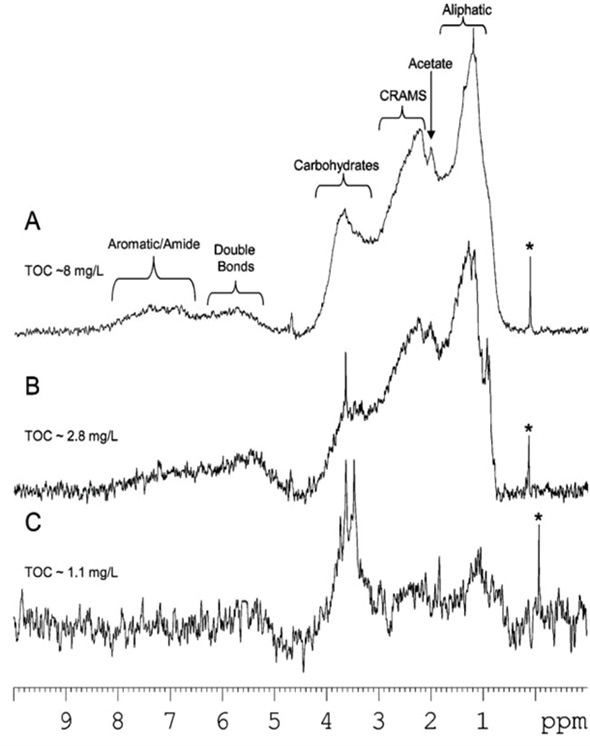
Figure 2. River, lake and ocean dissolved organic matter at natural abundance
Figure 3 shows the molecular characterization of the DOM of arctic ice. A large number of tiny molecules including short chain acids and amino acids are found and 80% of the total organic carbon (TOC) can be accounted for at natural abundance. Plants, tissues, soils and other more complex environmental samples are difficult to analyze using traditional NMR spectroscopy because such samples contain numerous physical states. Therefore, innovative NMR technology is needed.
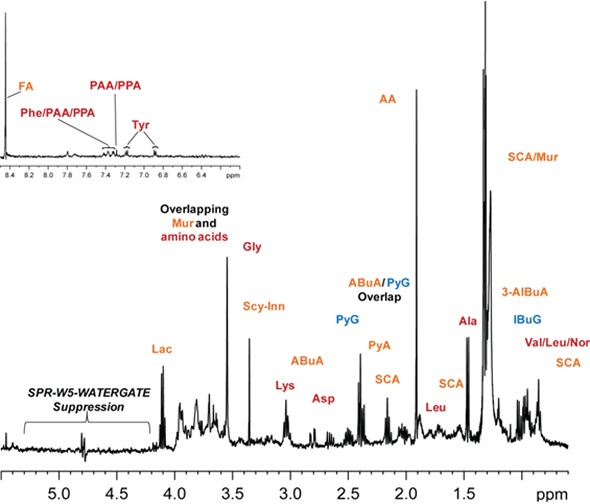
Figure 3. Molecular characterization of the arctic ice dissolved organic matter
Natural Multiphase Samples
Bruker has collaborated with the Environmental NMR Center (University of Toronto) to develop comprehensive multiphase NMR or CMP-NMR, which integrates the capabilities of liquid-state, solid-state and gel-state NMR into one approach. This technology makes it possible to detect and differentiate all liquids, solutions and gels in intact, unchanged samples in their natural state. Figure 4 shows the analysis of intact 13C labelled seeds and Figure 5 shows the capabilities of analyzing dynamic processes. During germination, it was possible to track the conversion of lipids to carbohydrates and to identify a wide range of structural and metabolic alterations during growth.
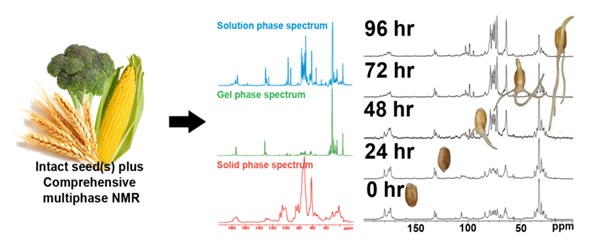
Figure 4. Analysis of intact 13C labelled seeds
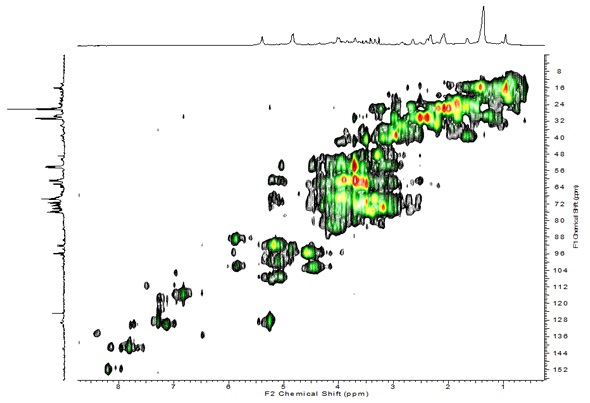
Figure 5. Capabilities of analyzing dynamic processes
Multidimensional CMP-NMR enables detailed structural assignments. In Figure 5, Arabidopsis seedlings were analyzed in their natural state without pre-treatment. Two dimensional analysis helps resolve overlapping peaks, enabling molecular-level characterization and peak assignments. In general, CMP-NMR has a wide range of applications in environmental science. It removes the need for pre-treatment/extraction and allows holistic study of the environmental samples. This technology shows great potential for studying in-situ molecular interactions.
Soil Structure
Limitations in analytical instrumentation have made it difficult to study the structure and composition of soils. Using CMP-NMR spectroscopy, it is possible to analyze soil in its native state, meaning chemical composition and molecular interactions between soil components can be understood.
A 2D heteronuclear experiment was used to compare an overlay of four key soil components including protein, lignin, carbohydrates and aliphatics (Figure 6) in soil with a whole soil sample. All major peaks in soil are accounted for in the overlay. Using different solvent systems (Figure 6), it is possible to establish how these components interact with one another. While lignin and proteins are water inaccessible, polar aliphatic groups and carbohydrates orientate towards the water. Lignin and proteins become available when hydrogen bonds are disrupted. A large amount of the protein in soil seems to be inside microbial cells.
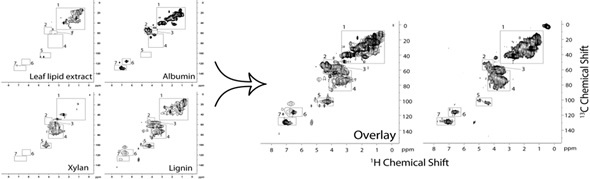
Figure 6. 2D heteronuclear experiment performed to compare an overlay of four key components
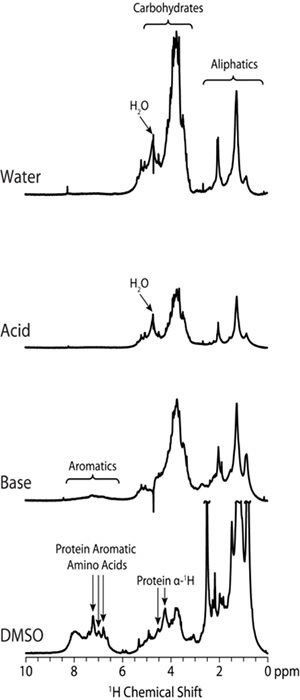
Figure 7. Different solvent systems used to determine interaction of components with each other
Molecular Interactions
CMP-NMR spectroscopy can be used to analyze samples in their intact state and is ideal for researching molecular interactions. In this case, an innovative experiment referred to as Reverse Heteronuclear Saturation Transfer Difference is employed to examine the binding of contaminants in whole soil. This works by passing saturation from the 19F found in the contaminant to the 1H protons in the soil organic matter in close proximity (Figure 8).This provides an NMR spectrum of only the fraction of organic matter that binds to a contaminant. PFOA selectively binds to soil protein, whilst perfluoronaphthol shows preferences for lignin (Figure 9).
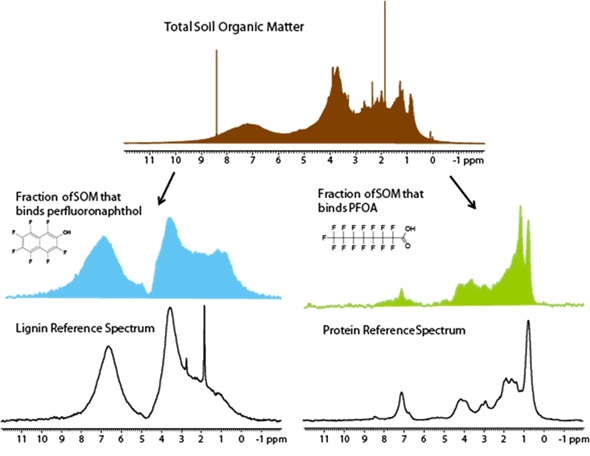
Figure 8. Reverse heteronuclear saturation transfer difference spectra
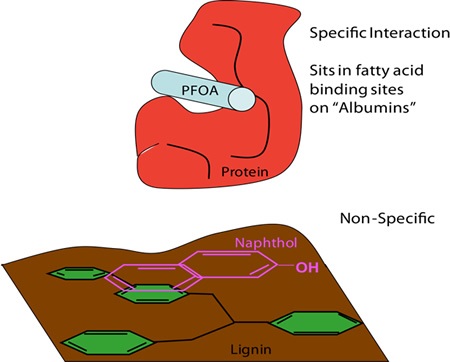
Figure 9. PFOA bind selectively to soil protein, whereas perfluoronaphthol shows preferences for lignin
In Vivo NMR Spectroscopy
In order to understand real-time biomolecular stress, it would be best to analyze organisms in vivo. To that end, a new technique has been designed to analyze Daphnia magna in a 5mm-NMR tube using a flow system (Figure 9) developed in house. 13C labelling with labelled algae enables multidimensional analysis in the liquid state, A less stressful environment is provided by solution NMR-based studies, but these are limited to analyzing relatively mobile chemical constituents (mobile gels and solutions).
CMP-NMR spectroscopy was used to analyze Hyalella azteca, making it possible to ascertain the molecular composition of all the components ranging from solution through to solids, within a living organisms for the first time (Figure 10) by NMR. Although spinning is stressful for the organism, it enables contaminants to be detected in all phases in vivo and also makes it possible to correlate the state of the contaminant with both metabolic and structural changes such as shell thickness and protein and lipid membrane disruption.
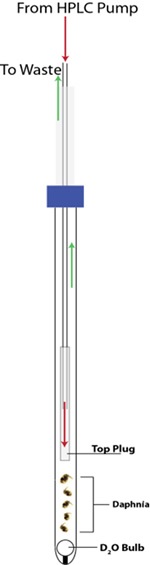
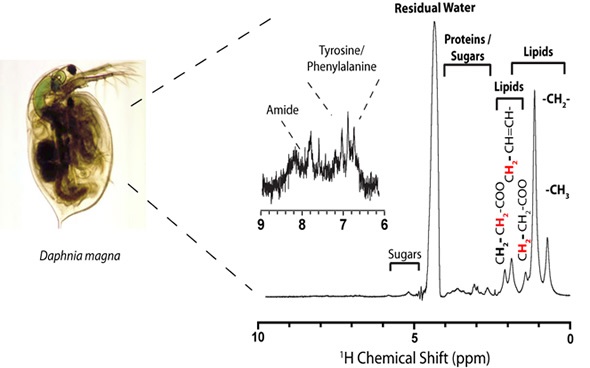
Figure 9. New technique used to analyze Daphnia magna in a 5mm-NMR tube
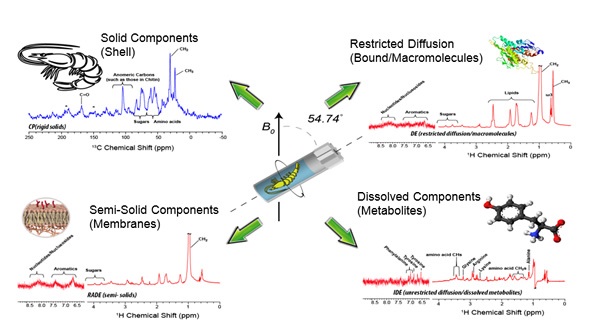
Figure 10. Using CMP-NMR spectroscopy to study Hyalella azteca
Conclusion
Detection of cellular response in relation to contaminant exposure allows policy makers to direct their attention towards treatment, remediation and prevention efforts for certain problematic target chemicals. In vivo data is of physiological significance, enabling researchers to examine the contaminants’ toxic mode-of-action and relate metabolic flux to protein and genetic expression, which are fundamental in a range of diseases.
References
- Lam and Simpson. Analyst. 2008. 133. 263-269.
- Pautler et al. Environ. Sci. Technol.2011. 45:11. 4710-4717.
- Courtier-Murias et al. J. Mag. Res. 2012. 217. 61-76.
- Lam et al. J. Agric. Food Chem. 2014. 62 (1). 107–115.
- Wheeler et al. The Plant Journal, 2014, in prep.
- Masoom et al. Nature Geosci. In prep.
- D’eon et al. Environ. Toxicol. Chem. 2010.29:8 1678-1688.
- Longstaffe et al. Environ. Sci. 2010.44:14. 5476-5482.
- Liaghati et al. Nat. Methods. In prep.
- Akhter et al. Preliminary data.
Acknowledgement
Produced from content authored by Hussain Masoom1, Ronald Soong1, Brent Pautler1, Buuan Lam1, Denis Courtier-Murias1, Leayen Lam1, Yalda Liaghati1, Mohammad Akhter1, Blythe Fortier-McGill1, Werner E. Maas2, Michael Fey2, Howard Hutchins2, Rajeev Kumar3, Martine Monette3, Henry J. Stronks3, Myrna J. Simpson and André J. Simpson1
- Department of Chemistry, University of Toronto, 1265 Military Trail, Toronto, ON, Canada, M1C 1A4
- Bruker BioSpin Corp., 15 Fortune Drive, Billerica, Massachusetts, USA, 01821-3991
- Bruker BioSpin Canada, 555 Steeles Avenue East, Milton, ON, Canada, L9T 1Y6
About Bruker BioSpin - NMR, EPR and Imaging

Bruker BioSpin offers the world's most comprehensive range of NMR and EPR spectroscopy and preclinical research tools. Bruker BioSpin develops, manufactures and supplies technology to research establishments, commercial enterprises and multi-national corporations across countless industries and fields of expertise.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.