Angiogenesis refers to the formation of new blood vessels in the body. As an important component of several disease states as well as normal physiological processes, angiogenesis is a complex, multi-step process that involves interactions between different types of cells and many growth factors.
Angiogenesis involves two main methods of vascular expansion by which the supply of nutrients to tissues is modified to match physiological demand. One method is the formation of new capillaries from existing blood vessels and the second is called vasculogenesis, which is the de novo production of blood vessels. Angiogenesis accompanies the growth and regeneration of organs, while vasculogenesis is a critical part of embryonic development.
The iBox® Explorer imaging microscope system (Figure 1) from Analytik-Jena is an effective tool for in vivo imaging of angiogenesis that allows researchers to understand and assess the various conditions that promote or prevent angiogenesis in human disease and preclinical models.

Figure 1. iBox Explorer imaging microscope
The system features a high resolution cooled CCD camera, designed to detect low fluorescent signals in the angiogenic process across a broad spectral range within living animals. In this article, the Analytik-Jena iBox Explorer is used to observe angiogenesis vessels in a murine model of liver cancer.
Materials and Methods
Vector, Cell Line and Animal
GFP-tagged plasmid was used to transfect the Hepatocellular Carcinoma Cell Line (HepG2). Cell culture and selection of GFP-expressing HepG2 cells were carried out. Male nude mice (BALB/c-nu/nu), aged six weeks, were supplied by Beijing HFK Bio-Technology, China and acclimated for seven days. The GFP-expressing HepG2 cells were harvested and kept cold. The nude mice were subcutaneously injected with cells (1 × 107 cells in 0.1mL phosphate buffered saline).
Orthotropic Transplantation Tumor Model
After a period of three weeks following HepG2-GFP inoculation, the mice that had cancer growths were used for orthotropic transplantation. Tumor tissues were collected and cut into pieces under aseptic conditions. The pieces of tumor were orthotopically transplanted into the liver lobes and the mice were caged and raised under specific pathogen free (SPF) conditions.
Fluorescent Microscopy
After a month following tumor implantation, the mice were anesthetized and the iBox Explorer was used to capture images. The system was configured using the following features:
- Motorized platform with built-in warming plate set to 37°C
- Automated BioLite™ MultiSpectral Light Source
- 3.2MP OptiChemi 610 camera
- GFP excitation and emission filter sets
- VisionWorks® LS Acquisition and Analysis software
The built-in magnification optics vary between 0.17x and 16.5x, thereby providing a broad range of imaging magnification options for this type of application.
Results and Discussion
The iBox Explorer was used to image GFP-fluorescence signals in the nude mice at different magnification levels, as shown in Figure 2 and 3. The GFP signals were not only detected in the transplant site, but in various regions of the bodies, indicating that the tumor cells had spread.
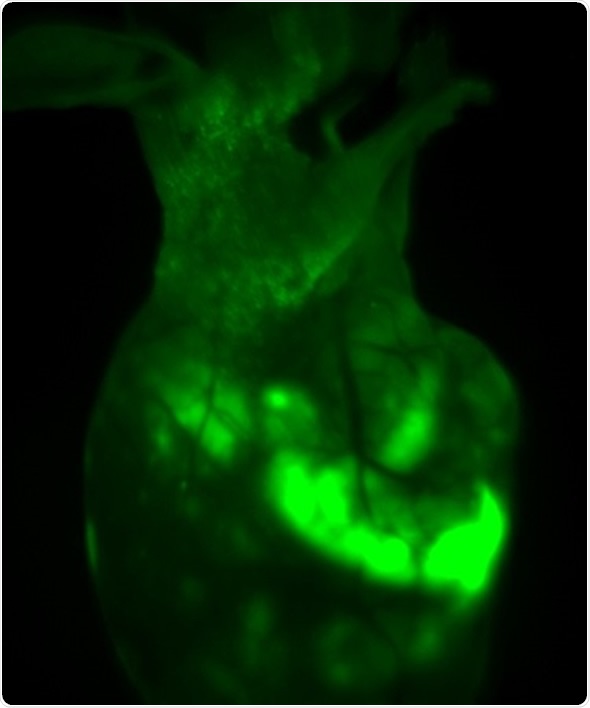
Figure 2. In vivo GFP fluorescent imaging of mouse transplanted with HepG2-GFP driven tumor. The image shows the mouse one month after orthotropic transplanted tumor in liver lobes, with survival and migration of transplanted tumor cells.
In addition, Images were captured of angiogenic vessels surrounding the tumor. The images of the GFP-fluorescent tumor signals obtained at 0.5x magnification are shown in Figure 3 (A), which corresponds to a 30mm2 field of view. Higher magnification images were captured to view the angiogenic vessels around the tumors. Figures 3(B), 3(C) and 3(D) correspond to the regions with the same names labeled in Figure 3(A). Vessels were then viewed at 2.5x and 1.66x magnifications, which corresponds to 6mm2 and 9mm2 fields of view, respectively. The angiogenic vessels are highlighted by yellow dashes.
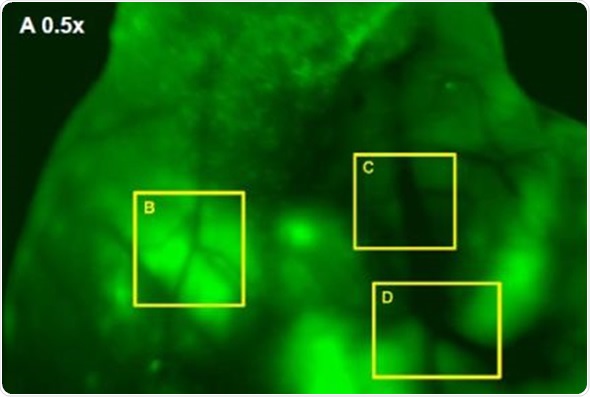
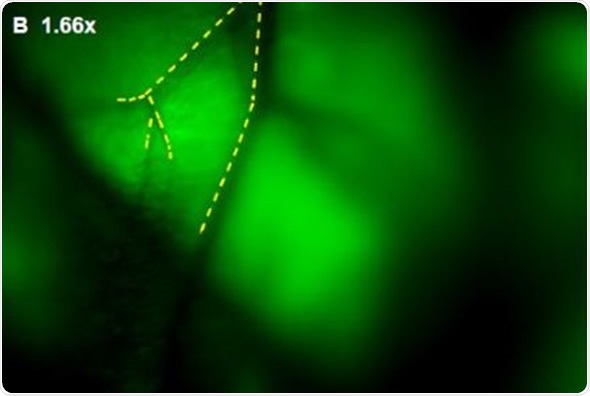

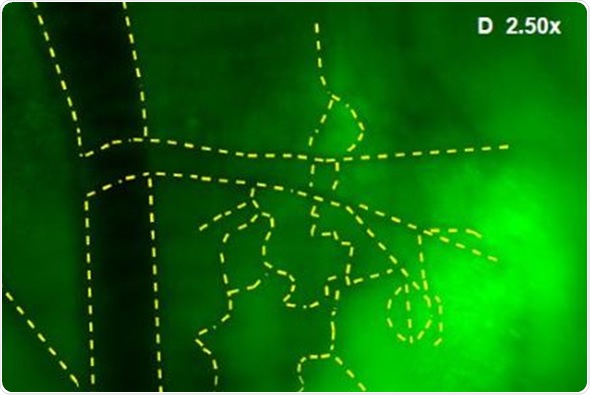
Figure 3. (A) GFP-Fluorescent signals were imaged with iBox Explorer at 0.5x magnification corresponds to a field of view of 30mm2 window. Areas with angiogenic vessels around tumor were marked. Figs. 3 (B, C, D) correspondent areas with angiogenic vessels were imaged at 1.66x or 2.50x magnifications, which correspond to fields of view of 9mm2 or 6mm2 respectively. Angiogenic vessels were marked with yellow dashes.
Conclusion
After a month following tumor implantation, strong fluorescence signals were captured in the mice. The tumor cells had spread from the liver lobes to other tissues in the body.
In order to examine the angiogenic responses, the angiogenic vessels surrounding the cancerous tissues were magnified and captured with higher resolution imaging, and for further analysis, the numbers and lengths of the vessels were determined and recorded. The study proved that the mouse model of HepG2-GFP liver cancer serves as a reliable and reproducible system for studying angiogenesis.
The iBox Explorer imaging microscope is an important tool that can be used for preclinical in vivo fluorescence imaging of angiogenesis, providing high quality imaging for the monitoring of vessel changes.
This method can be applied in both quantitative and qualitative evaluation of many angiogenesis studies and in the monitoring of anti-angiogenic treatments, so as to investigate angiogenic responses to specific perturbations of the angiogenic process or to gain insight into regulatory pathways, for example. Analytik-Jena’s iBox Explorer imaging microscope provides a simple, fast and more accurate technique for vessel imaging in murine models.
References
- Imaging tumour angiogenesis, Cancer Imaging. 2005; 5(1): 131–138.
- Challenges for imaging angiogenesis, British Journal of Radiology (2001) 74, 886-890
About Analytik Jena US
 Analytik Jena is a provider of instruments and products in the areas of analytical measuring technology and life science. Its portfolio includes the most modern analytical technology and complete systems for bioanalytical applications in the life science area.
Analytik Jena is a provider of instruments and products in the areas of analytical measuring technology and life science. Its portfolio includes the most modern analytical technology and complete systems for bioanalytical applications in the life science area.
Comprehensive laboratory software management and information systems (LIMS), service offerings, as well as device-specific consumables and disposables, such as reagents or plastic articles, complete the Group’s extensive range of products.
About Life Science
The Life Science product area demonstrates the biotechnological competence of Analytik Jena AG. We provide a wide product spectrum for automated total, as well as individual solutions for molecular diagnostics. Our products are focused to offer you a quality and the reproducibility of your laboratory results.
This will surely ease your daily work and speed up your work processes in a certain way.
All together we support you through the complete process of the lab work. Besides we offer customized solutions and are able to adapt our products to your needs. Automated high-throughput screening systems for the pharmaceutical sector are also part of this segment’s extensive portfolio.
About Analytical Instrumentation
Analytik Jena has a long tradition in developing high-performance precision analytical systems which dates back to the inventions made by Ernst Abbe and Carl Zeiss. We have grown to become one of the most innovative manufacturers of analytical measuring technology worldwide.
Our business unit Analytical Instrumentation offers excellent competencies in the fields of optical spectroscopy, sum parameters and elemental analysis. Being proud of our core competency we grant all our customers a long-term warranty of 10 years for our high-performance optics.
About Lab Automation
With more than 25 years of market experience, Analytik Jena with its CyBio® Product Line is a leading provider for high quality liquid handling and automation technologies. In the pharmaceutical and life science industries, our products enjoy the highest reputation for precision, reliability, robustness and simplicity.
Moreover, the Automation Team designs, produces and installs fully automated systems tailored to our clients' application, throughput and capacity requirements. From stand-alone CyBio® Well up to fully customized robotic systems we handle your compounds, biomolecules and cells with great care.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.