The examination of microbial populations is frequently performed in substantial quantities, thereby overlooking the variations that exist at the individual cellular level. To help address this issue, microfluidic encapsulation provides a high-throughput approach to analyzing single-cell populations.
The CYTRIX hydrogel kit has been specially developed to ensure the high viability of encapsulated cells and optimal gelation for use in microfluidic devices.

Figure 1. CYTRIX Microfluidic Hydrogel Kit, consisting of CYTRIX hydrogel solutions, the Pico-GenTM double aqueous biochip, and Pico-SurfTM surfactant. Image Credit: Sphere Fluidics
The CYTRIX Hydrogel Kit is an all-in-one system for microfluidic single-cell hydrogel encapsulation. This convenient kit consists of the innovative CYTRIX hydrogel, Sphere Fluidics' Pico-Gen™ double aqueous biochip, and Pico-Surf™ surfactant (as depicted in Figure 1).
The CYTRIX hydrogel technology is based on the highly successful CLEX hydrogel system, which efficiently encapsulates a wide range of cells, including mammalian cells, bacteria, and other microorganisms, with a leading cellular compatibility rate of approximately 95% viability across various cell types.1
The CLEX formula ensures that cells are protected during gelation, which takes place at physiological pH and temperature, and also guarantees clog-free microfluidic operations to produce uniform hydrogel beads.
Additionally, the hydrogel offers the option for reversible gelation, enabling encapsulated cells to be released at any desired time.
To showcase the efficacy of the CLEX technology for the efficient encapsulation and examination of microbes, microbeads were immobilized onto surfaces pre-patterned with poly(ethyleneimine) (PEI) and were evaluated for viability. Clones were then selected and expanded from individual microbeads.
Aims & objectives
- This study2 examined the use of CLEX hydrogel technology to generate monodisperse droplets using a double aqueous system, with downstream use in a microarray for clonal selection.
- Multiple microorganisms were encapsulated to confirm the viability of different populations in the alginate hydrogel.
- Demonstrate the potential of CLEX hydrogels for encapsulation, culture, and downstream selection or assay of viable cells.
Methods and results
CLEX hydrogel microfluidic encapsulation and array workflow
- CLEX solutions containing microorganisms are introduced into a double aqueous microfluidic chip along with a carrier fluid to create hydrogel beads.
- Bead emulsions are broken, leaving purified bead suspensions.
- Beads are immobilized on the micropatterned glass to form a microarray.
- Encapsulated cells are cultured/incubated and observed for growth and other cellular measurements.
- Clones of interest are selected, beads dissolved, and culture-expanded.
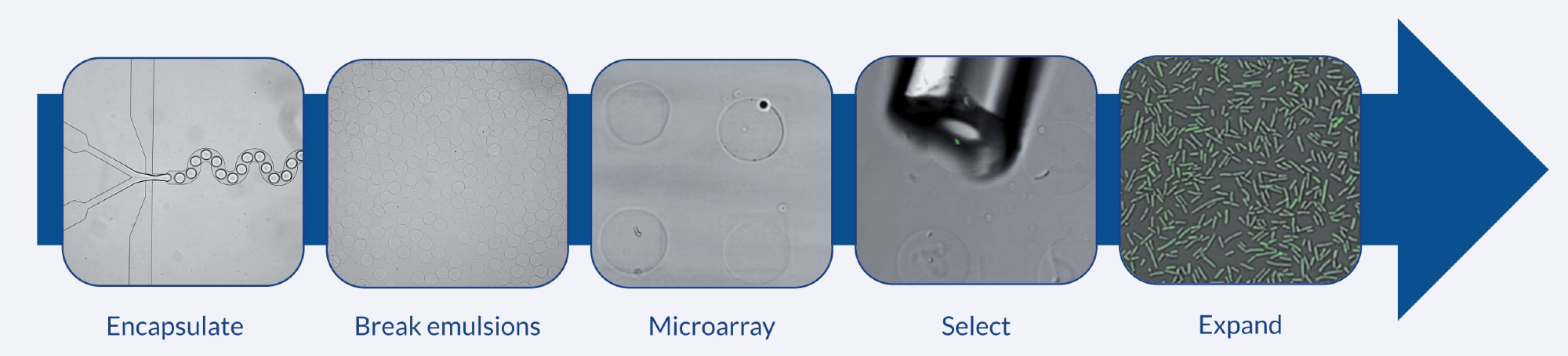
Figure 2. Workflow for array of alginate microbeads. Bacteria (P. putida) are encapsulated in CLEX hydrogel droplets and deposited on a PEI patterned surface to create an array. The clone of interest is selected and expanded. Image Credit: Sphere Fluidics
Encapsulation of microorganisms in alginate beads
CLEX, or Competitive Ligand Exchange X-linking, is a patented gelation technology that uses ionic crosslinking to form alginate polymers at physiological pH. The process of gelation begins with mixing two aqueous precursor solutions, referred to as Solution A and Solution B.
Solution A consists of alginates and a gelling ion, such as Ca2+ or Mg2+, that is chelated by a gelling ion chelator, like EDTA2- or CDTA2-. Solution B contains alginate and an exchange ion, such as Zn2+, Fe2+, or Mn2+, that is chelated by an exchange ion chelator, such as EDDA2- or glycine1-.
The exchange ion binds to the gelling ion chelator in Solution A, releasing the gelling ion and facilitating the crosslinking of alginate to form a hydrogel. This internal gelation method leads to evenly distributed gelling ions within the material, resulting in homogeneously crosslinked gels.
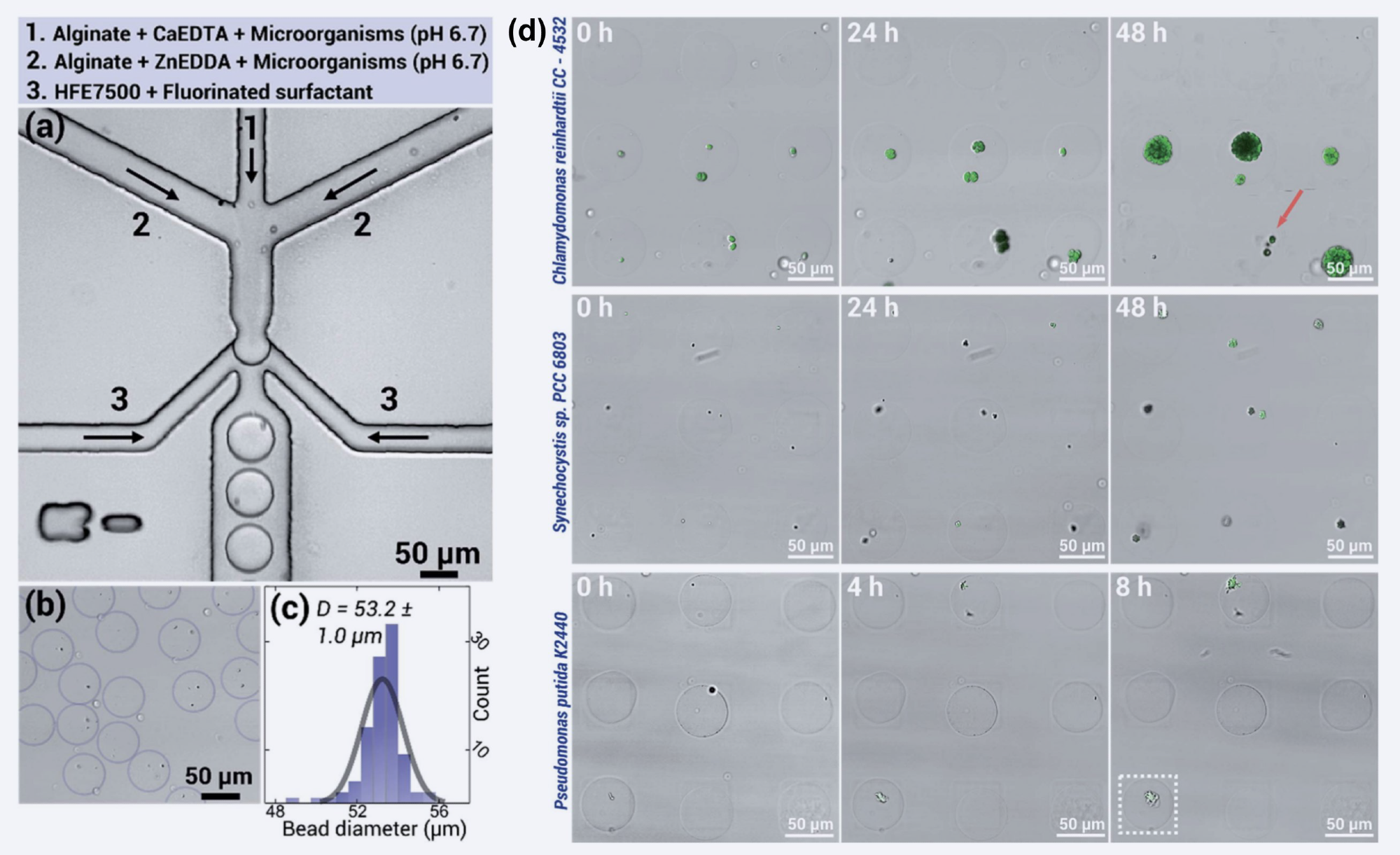
Figure 3. Using a double-aqueous microfluidic chip (a), microbes can be easily encapsulated in beads (b) of uniform size (c). The proliferation of three different microbial populations after encapsulation was monitored over time. Images include merged signals from brightfield and fluorescence imaging. (d) Top row: Chlamydomonas reinhardtii CC-4532 (chlorophyll), middle row: Synechocystis sp. PCC 6803 (chlorophyll), bottom row: Pseudomonas putida KT2440 (GFP-expression). Figure reproduced from Håti et al. 2016 with permission from Royal Society of Chemistry.
Using this method in double aqueous microfluidic chip results in monodisperse (Figure 3b) and uniform-sized beads with a mean diameter of 53.4 um ± 1.0 um (Figure 3c). The uniformity and homogeneity of the beads make them ideal for use in microfluidic single-cell encapsulation and analysis.
The pattern used to immobilize the microorganisms in the hydrogel beads was created using a process called microcontact printing. Glass slides were treated with polyethylene glycol (PEG) and then patterned with poly(ethyleneimine) (1% wt) (PEI) through the use of PDMS stamps.
The interaction between the PEI and alginate in the hydrogel beads, which is due to the opposing charges, leads to the high viability of the encapsulated microorganisms when applied to the microarray.
The growth rates (Figure 3d) of these microorganisms are comparable to those of unencapsulated cultures, demonstrating that this technology results in normal growth and development.
References
- Håti, Armend G., et al. "Versatile, cell and chip friendly method to gel alginate in microfluidic devices." Lab on a Chip 16.19 (2016): 3718-3727.
- Håti, Armend G., et al. "Microarrays for the study of compartmentalized microorganisms in alginate microbeads and (W/O/W) double emulsions." RSC advances 6.115 (2016): 114830-114842.

 Download the full paper
Download the full paper
About Sphere Fluidics
Our vision
Our philosophy is simple. We combine our knowledge and resources to help you find rare and valuable biological variants, while helping you to save time, reduce costs and stay a step ahead of the competition.
Our novel single cell analysis systems offer the rapid screening and characterization of single cells. These systems are underpinned by our patented picodroplet technology, specifically designed to increase your chances of finding that rare ‘one-in-a-billion’ molecule or cell that could be an industry blockbuster.
We understand that time is of the essence. That’s why our technologies boost throughput and assay sensitivity across a range of applications. Most importantly, our flexible systems evolve alongside your changing research needs, providing an adaptable platform that helps you to meet your goals.
Our history
Founded in 2010, Sphere Fluidics is an established Life Sciences company, originally spun out from the University of Cambridge. We initially developed 25 patented products – biochips and specialist chemicals – which currently assist hundreds of customers globally with their research.
We initially focused on producing novel biochip systems and providing R&D services. We have since extended our expertise and are developing a technology platform that enables discovery in a range of growing markets through single cell analysis. Our systems make the development of new biopharmaceuticals faster and more cost-effective, improve monoclonal antibody screening, cell line development, and overall research efficiency in a number of other applications including synthetic biology, single cell diagnostics, prognostics and single cell genome editing.
The Cyto-Mine® Single Cell Analysis System is our flagship product – the first integrated, benchtop system to automatically analyse, sort and dispense millions of individual cells in just a single day.
Our partnerships
We value and are always open to discussing new collaborative, successful and innovative academic and industry partnerships to further develop and improve our single cell technologies.
Our Technology Access Programmes and Collaborative Services exist to enable academic researchers and companies alike to tap into our application-specific expertise through direct partnerships.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.