Sponsored Content by SepmagReviewed by Maria OsipovaMay 9 2024
Magnetic bead separation is a fast, effective, and clean process that scientists use as an alternative to filtration and centrifugation techniques for separation.
Magnetic beads and particles can be functionalized with various biomolecules such as antibodies, antigens, proteins, catalyzers, or nucleic acids. This allows them to bind cells, bacteria, viruses, and a diverse range of other biological entities.
Magnetic bead separation allows for the separation of complexes of magnetic beads and their bound materials from a complex mixture in solution using a single magnetic separation rack. The outcome is an isolated solution of the target biological elements that can be enriched and concentrated through this procedure.
Characterizing and parameterizing the process performed by magnetic beads requires a significant amount of effort.
When selecting magnetic beads, it is important to consider various factors such as their diameter, magnetic pigment content, density, surface activation (plain, covalent, bio-functionalized), and even acceptable parameter variations.
Another crucial factor to consider for the magnetic bead separation technique is the magnetic separator, which effectively separates the beads from the solution.
An external magnetic force is essential to manipulate the beads, commonly referred to as a magnetic separation rack. Optimizing magnetic beat separation processes involves determining the most suitable specifications for both the magnetic beads and the magnetic separation rack.
A key parameter that defines the magnetic bead separation process is magnetic force. There are two major forces that play in your solution:
- The drag force is generated by the viscosity of the solution, which prevents the molecules from separating.
- The magnetic force of the magnetic separator rack is powerful enough to overcome the drag.
For efficient magnetic bead separation, it is critical to ensure that the separation conditions (magnetic force) are constant across the entire working volume. Therefore, it is crucial to guarantee that the separation rack can adapt to the working volume.
Many conventional separation racks are only efficient for a small amount of solution and are not designed for magnetic separation of larger volumes. They also struggle to scale to larger quantities of solution.
If the working volume changes between batches and the magnetic force is unevenly distributed in the sample, considerable issues are likely to occur with batch-to-batch consistency. This is evident through extended separation times, loss of magnetic beads, and issues with irreversible aggregation/clumping, among other problems.
Magnetic Bead Separation Systems: Maintaining lot consistency
Ensuring lot-to-lot consistency becomes challenging without a precisely calibrated magnetic separator rack, raising concerns about achieving in-lot consistency when the homogeneity of magnetic bead separation conditions in the working volume is uncertain.
This issue emerges when handling samples at a small scale but might be obscured by the inherent variability of magnetic beads. However, it becomes pronounced when the magnetic bead separation process encompasses various working volumes—such as during validation across different scales or when production volumes exceed those of the final application.
At these points, challenges manifest in the form of prolonged separation times, notable losses of magnetic beads (and biomolecules), and the onset of irreversible aggregation issues, leading to the formation of clumps.
These issues are often just the result of insufficient specification in the magnetic force profile used for the magnetic bead separation. Unfortunately, in most instances, individuals are not even aware of the importance of considering this factor.
To address these problems, it is important to consider the key parameter in defining a magnetic bead separation process, which is magnetic force. Magnetic force determines the magnetic bead separation speed by working against the opposition of the drag force generated by buffer viscosity.
Magnetic force: Key parameter defining magnetic bead separation
The fundamental principle of magnetic force dictates that homogeneous magnetic fields produce magnetic torque rather than magnetic force.
In essence, regardless of the magnetic force intensity, the beads will not move linearly if there is no gradient present; instead, they will simply rotate to align their magnetic moments with the field. Such rotation will not aid in the separation of your biological materials.
To produce a magnetic force, it is necessary to have a magnetic force that varies with distance (a magnetic force gradient). A strong permanent magnet is often needed for magnetic bead separation because it produces a strong magnetic force gradient, not because it has a strong magnetic field.
A closer look at the formula shows that two important variables influence magnetic force:
- The magnetic moment of the beads
- The profile of the magnetic field
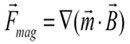
Based on the magnetic properties of the beads, their magnetic moment either changes in a linear manner with the applied magnetic field (assuming the susceptibility remains constant) or, if the field is sufficiently strong, the magnetic moment of the bead remains constant (indicating magnetic saturation).
Upon rewriting the magnetic force expression for both magnetic behaviors, it becomes evident that the dynamics of the beads are different.
- If the magnetic bead possesses a magnetic moment that fluctuates (i.e., a linear response with a constant susceptibility), it will encounter a force that is directly related to the gradient of the square of the magnetic field.
- When saturated (with a constant magnetic moment), the bead undergoes a force that is directly proportional to the gradient of the magnetic field.
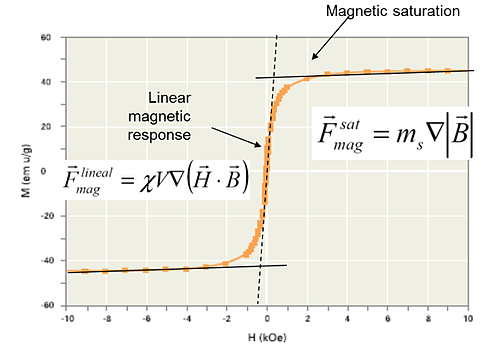
Image Credit: Sepmag
A constant magnetic force can be achieved if the conditions for magnetic material saturation and a constant magnetic force gradient are concurrently met.
Having defined the magnetic bead separation process, this next section of the article will delve deeper into the dynamics of the process.
Bead behavior in magnetic bead separation
The basic assumption is that every magnetic bead moves independently. To validate this scenario, it is necessary to compute the theoretical speed of magnetic bead separation.
This involves determining the magnetic moment, magnetic field gradient, and buffer viscosity to calculate the separation velocity. Subsequently, the separation time for the farthest bead can be predicted by straightforwardly dividing the distance by the speed.
Image Credit: Sepmag
The formula can be tested by comparing experimental results. The light traversing the suspension can be quantified using a cylindrical vessel and a Sepmag separation rack that produces a radial magnetic field gradient.
As the separation progresses, the solution will transition from being cloudy to becoming clear as the magnetic particles move along the magnetic field gradient and gather at the outer edges of the vessel.
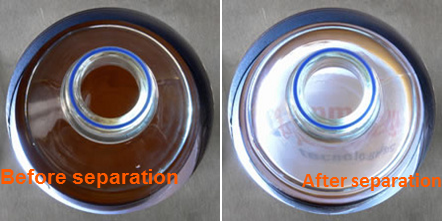
Image Credit: Sepmag
The experimental results below demonstrate excellent agreement with the model for the small-diameter magnetic particles used in this experiment. However, the magnetic bead separation process takes one day to complete.
This theory accurately predicted the separation time and the impact of increasing the magnetic force gradient. However, for many life science applications like CLIA immunoassay, using these small magnetic beads would be impractical due to the need for faster separation times, typically a few seconds.
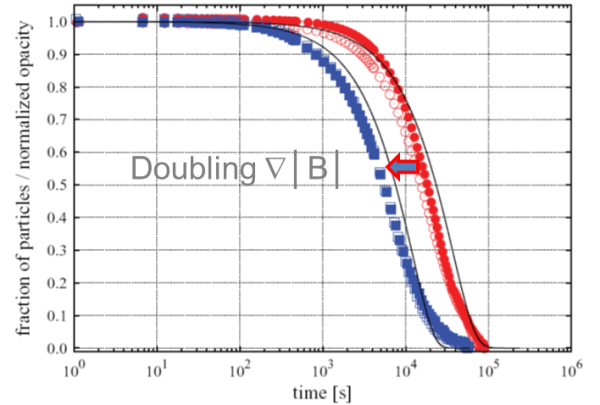
Image Credit: Sepmag
The same calculations for suspensions with larger magnetic beads can be performed using a similar magnetic force gradient. In this scenario, the predicted separation times are significantly shorter than experimental values. This experimental separation was accomplished in less than three minutes, which is much faster than the anticipated one-hour separation time.
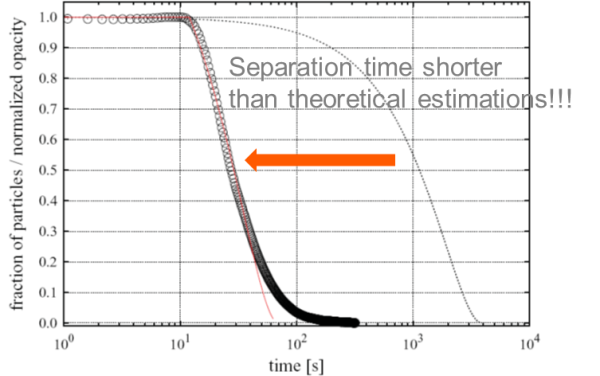
Image Credit: Sepmag
So what is happening here? The explanation is quite straightforward: Magnetic beads, commonly used in many life science applications, do not behave like typical particles.
Chain-like aggregation of magnetic beads improves separation time
When a magnetic field is applied to a suspension of magnetic beads with a large enough diameter, the beads become magnetized and exhibit small magnet-like behavior. They align with one another, forming chain-like structures.
These clusters resemble the movements of very large ‘beads’ but travel at a higher speed than single magnetic beads. It is worth noting that the chains are created in the direction of the magnetic field, but their movement is along the magnetic field gradient direction.
Generation of magnetic beads chain-like structure when a magnetic field is applied
Video Credit: Sepmag
When the magnetic beads are superparamagnetic, their magnetic moment becomes zero, and the chains dissolve once the magnetic force is removed, such as when the vessel is moved out of the magnetic bead separation device.
Dissolution of magnetic beads chain-like structures when magnetic field is removed
Video Credit: Sepmag
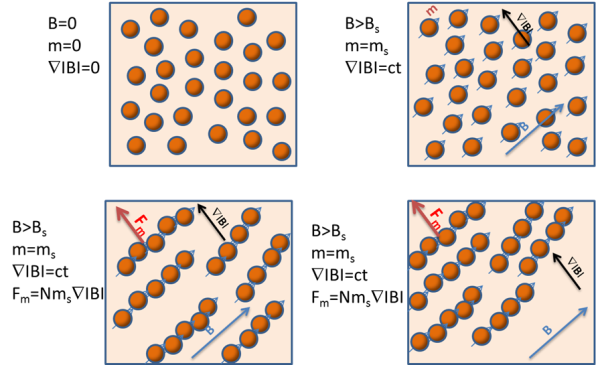
Image Credit: Sepmag
The practical consequences of this collective behavior are significant. The concentration of the beads in suspension has an important impact on the separation time, as chain-like structures are vital for the magnetic bead separation process.
It is important to take into account bead concentration when developing a magnetic bead separation protocol. As the concentration increases, the magnetic bead separation becomes faster. When the beads are closer, they can easily and quickly form chains.2
After defining the magnetic bead separation conditions, it was discovered that the beads can work in isolation or cooperatively. The separation times can vary significantly depending on this, irrespective of the magnetic separation rack used.
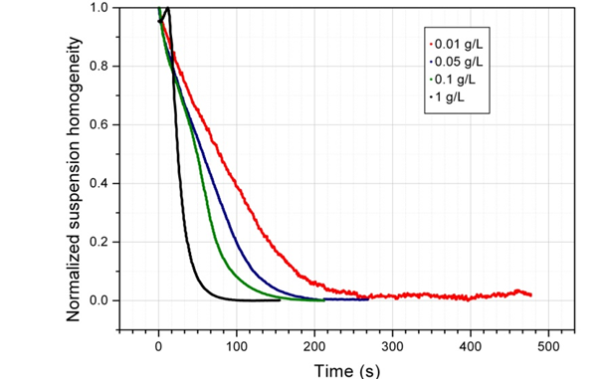
Image Credit: Sepmag
How can we determine if magnetic beads are acting cooperatively? By using a homogenous magnetic beat separation system and closely monitoring the process, researchers from UAB and ICMAB have developed models and tested them in collaboration with Sepmag R&D staff.3
An expression for the average length of the chains, N*, has been defined. If N* is greater than 1, it indicates cooperative behavior. When N* is well below 1, the magnetic beads separate into individual particles.

The value of N* varies with the square root of the concentration and is influenced by Γ, which is the ratio between the dipole-dipole magnetic energy and the thermal energy.
If the value of N* is plotted according to the diameter for fixed magnetic pigment content, it can be observed that by increasing the concentration, small magnetic beads can be separated.
The important parameter is bead size (note the logarithmic scale for the Y-axis). Increasing magnetic pigment content (or its magnetization) could be a viable approach, but it usually leads to an increase in bead density and sedimentation ratio.
Excessive sedimentation can pose a challenge if the beads precipitate to the bottom of the vessel before magnetic separation can take place.
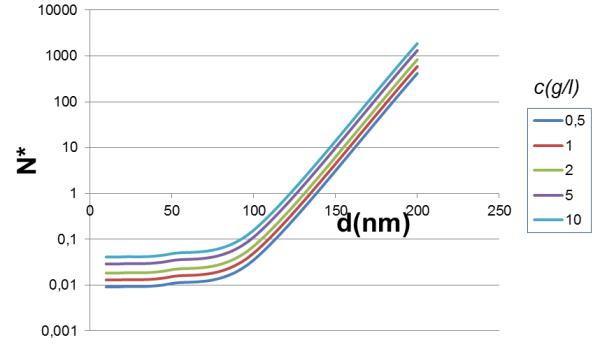
Image Credit: Sepmag
In conclusion, magnetic bead separation is much more complex than just placing a permanent magnet next to a test tube. The magnetic moment of the beads and the consistency of the magnetic field gradient are important factors for assuring separation speeds that are useful for bioscience applications.
Therefore, choosing a properly scaled magnetic bead separation rack is very important to ensure batch-to-batch consistency.
In addition, the bead diameter and magnetic pigment concentration influence the behavior of the beads during the separation process and are equally significant parameters when trying to achieve useful separation times.
For a cost-effective, rapid, consistent, and scalable process, it is crucial to have a well-defined magnetic bead separation process when working with sample sizes ranging from microliters to tens of liters.
References and further reading
- M. Benelmekki & Ll. M. Martinez “Magnetophoresis of iron oxide nanoparticles: A tool for synthesis monitoring and magnetic bead applications” “Drug Delivery and Nanomedicine” vol 5. Editor J.N. Govil, Studium Press LLC, USA (2013).
- J. Faraudo & J. Camacho (2010). Colloid Polym. Sci., 288:207
- J. S. Andreu et al, PHYSICAL REVIEW E 84, 021402 (2011)
About Sepmag 
Sepmag develops smart and scalable magnetic bead separation equipment for the international diagnostics market and for any user of magnetic bead separation techniques.
Sepmag's innovative Smart & Scalable Magnetic Bead Separators are designed to deliver unparalleled control and efficiency across all volumes, preventing bead aggregation, minimizing material loss, monitoring and keeping records for Quality Control purposes, and maximizing safety.
These benefits are applicable through a range of laboratory settings from R&D facilities to large scale production processes. Sepmag is based in Barcelona and sells in North America, Europe and Asia.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.