Sponsored Content by SepmagReviewed by Maria OsipovaMay 9 2024
Magnetic bead separation is widely used in the life sciences field. From cell sorting to molecular diagnostics, this technology can be used with a wide range of volumes, from a few nanoliters in lab-on-chip applications to tens of liters for the production of IVD reagents.
To monitor the separation process, scientists take advantage of the typically dark color of the magnetic bead suspension. As the solid phase migrates to the retention area during separation, the buffer clears, signaling the end of the process. This visual cue allows for monitoring by sight, and when greater precision is necessary, a spectrometer can be used.
One drawback of these approaches is that they only provide an analysis of the result, indicating whether the separation is complete after a specific time. Visual inspection alone does not provide any insight into the process or allow for the detection of faster-than-expected separations when the separation time is fixed.
Why monitor magnetic bead separation processes in R&D?
For technicians employing conventional magnetic separators, the information related to real-time time monitoring is very challenging to interpret. Due to the variability of the magnetic force and the gradient based on the position of the magnetic bead, analyzing changes in the process dynamics becomes difficult.
The magnetic behavior varies because changes in the suspension/beads are different at different points in the working volume, making the data very hard to interpret. However, when a magnetic bead separation system with constant magnetic force is employed, the data provided by real-time monitoring is much simpler to analyze.
Given that the applied magnetic force pattern remains constant across the working volume, the behavior of the magnetic beads is influenced directly by their magnetic moment and diameter—the latter impacts the drag force. Furthermore, changes in the buffer’s viscosity due to temperature or compositional fluctuations are readily observable, as these variations directly alter the speed of separation.
Continuous monitoring allows for the optimization of protocols by extrapolating curves and studying the interaction between the beads at different concentrations.
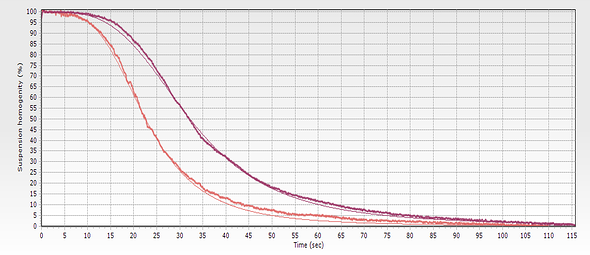
Image Credit: Sepmag
Why monitor magnetic beads separation production processes?
When the magnetic beads separation process is moved from the R&D laboratory to the production facilities, Quality Control becomes a significant concern.
Validation of the process settings is the initial problem, but when a constant magnetic force is applied, it becomes a non-issue. After the force has been tested in R&D or during the validation step, the production system is assigned similar values to ensure that there are no losses or irreversible aggregation issues.
Constant force systems allow for the adjustment of separation time to accommodate the new vessel diameter. Since the speed is directly related to the magnetic force, the time required for the farthest beads to reach the retention region is t = distance/speed.
In production processes, however, relying solely on separation time poses a risk of approving faster batches than those validated, as the suspension transparency may correct at the separation time.
For instance, if magnetic beads are clumped together before starting the separation, the batch may initially appear to be correct, but the resulting product could lead to quality issues in the later stages of the manufacturing process.
When monitored in real time, the separation process allows for the detection of several quality issues. These include bead aggregation, incorrect bead concentration, variations in bead diameter and magnetic charge, as well as changes in buffer viscosity, which may result from incorrect composition or temperature differences.
Image Credit: Sepmag
Although magnetic bead separation users have not traditionally monitored the process, there are compelling reasons to do so at both the R&D and production levels.
Historically, the main barrier to monitoring was the absence of controlled conditions. However, advanced magnetic bead separation systems that maintain a constant force throughout the working volume now overcome this limitation. This enhancement allows users to leverage the insights provided by real-time monitoring, significantly benefiting the process.
Monitoring magnetic beat separation processes options and choices
The standard approach for developing and validating magnetic bead separation processes involves sampling the supernatant at various time intervals. This sample is typically measured using a spectrophotometer.
The concentration of beads is determined by carefully selecting the right wavelength to prevent any interference from the biomolecules in the buffer. This wavelength is then compared with a calibration curve.
The optimal separation time is determined when the number of beads approaches zero. Recently, measuring magnetic susceptibility at the fundamental frequency or its harmonics has been proposed as an alternative method.
One issue with these approaches is the variation in concentration at different points of the working volume, which can be attributed to the sampling method used. The question of how and from where the sample should be taken is a topic of debate.
Having access to real-time information across the entire working volume—from milliliters in the laboratory to liters in production—is crucial for monitoring the process’ progress. Optical measurements are emerging as a preferable method for achieving this, as they allow for non-intrusive monitoring, unlike some magnetic techniques, which can interfere with the Magnetic Bead Separation Process.
The initial suspension containing homogeneously distributed magnetic beads is typically opaque unless the concentration is very low. However, as the solid phase separates, the buffer becomes transparent.
By measuring the light transmitted through the vessel, a real-time assessment of the evolution of the Magnetic Bead Separation Process can be obtained. When constant magnetic force is used, the beads move radially from the center, allowing for accurate observation of the process evolution through the measurement of transmitted light.
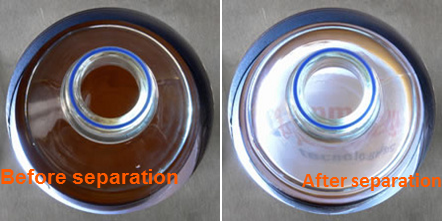
Image Credit: Sepmag
This method can be applied with various wavelengths, if required, by using spectrophotometry. Improving accuracy is possible, but it is important to customize the system for the specific bead/particle and suspension.
The wavelength required for a 50 nm magnetic particle differs from that of a 1-micron magnetic bead. A more straightforward method, measuring white light transmittance, is effective for a wide range of diameters and buffers.
With the constant magnetic force provided by Advanced Magnetic Bead Systems, it becomes simple to optically monitor the evolution of the process. These systems also make it easy to interpret the obtained data and use it to gain insights into the process and/or control its performance.
About Sepmag 
Sepmag develops smart and scalable magnetic bead separation equipment for the international diagnostics market and for any user of magnetic bead separation techniques.
Sepmag's innovative Smart & Scalable Magnetic Bead Separators are designed to deliver unparalleled control and efficiency across all volumes, preventing bead aggregation, minimizing material loss, monitoring and keeping records for Quality Control purposes, and maximizing safety.
These benefits are applicable through a range of laboratory settings from R&D facilities to large scale production processes. Sepmag is based in Barcelona and sells in North America, Europe and Asia.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.