Airway inflammation is a central process in asthma and other lung diseases1. Being able to measure this inflammation and monitor the patient’s medication is regarded as a gold standard in the management of respiratory diseases.
It is increasingly recognised that the measurement of FeNO, in particular reaction to constitutes, is a novel way to monitor separate aspects of diseases. These include asthma, COPD and interstitial lung diseases that are not assessed by other means, such as lung function2.
The production of nitric oxide is often found to be higher in inflammatory conditions such as asthma and can therefore be used for the detection and management of such conditions6. Nitric oxide measurement is not intended as a stand-alone method for diagnosis and should be used in conjunction with other evaluation methods and tests7.
Using FeNO measurements to evaluate airway inflammation in asthma represents a significant advance in respiratory medicine3, but until now this has been an expensive test to deliver in everyday practice.
The use FeNO measurements has also been proven to be one of the most accurate diagnostic methods when ruling in or out eosinophillic asthma (allergic asthma)4.
Benefits of performing FeNO tests:
- Non-invasive, quick and easy to perform3
- Shows patient’s response to treatment, enabling the correct prescription of medication
- Shows patient compliance
- Aids in identifying patients who do/do not require on-going treatment5
- Shown to be superior to the majority of conventional tests of lung function, such as peak flow recording and spirometry3
- Aids in differentiating between allergic (eosinophillic) and non-allergic asthma4.
Benefits of performing FeNO tests
FeNO tests provide the following benefits:
- Shows patient’s response to treatment and allows the right prescription of medication
- Fast, non invasive, and easy to perform3
- Demonstrates patient compliance
- Shown to be better than other traditional tests of lung function, such as spirometry and peak flow recording3
- Helps identify patients who do/do not need ongoing treatment4
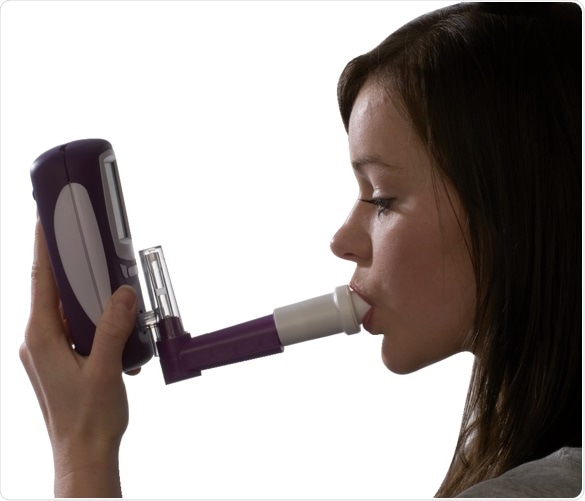
Image credit: Bedfont Scientific
NObreath® accessories
Each NObreath® comes with:
- 50 Mouthpieces – These are specifically designed with advanced bacterial filtration to eliminate 99.9% of airborne bacteria present in the patient’s breath5. The mouthpieces can be used up to three times by each patient, considerably lowering the cost of FeNO testing.

Image credit: Bedfont Scientific
|
Order code
|
Description
|
|
NTK50
|
50 mouthpieces and 1 NObreathFlo™
|
- NObreathFlo™: eye level flow indicator makes it easy to maintain a steady flow during exhalation, even for young children.
- The combined use of mouthpieces and NObreathFlo™ enables users to conform to ATS/ERS guidelines for FeNO testing2.
- Carry case: This protects the NObreath® when being transported or while in storage.
- 50 alcohol-free cleaning wipes: NObreath® or any of its components cannot be used with gels/wipes containing alcohol. Order code: WIPES

Image credit: Bedfont Scientific
NObreath® features
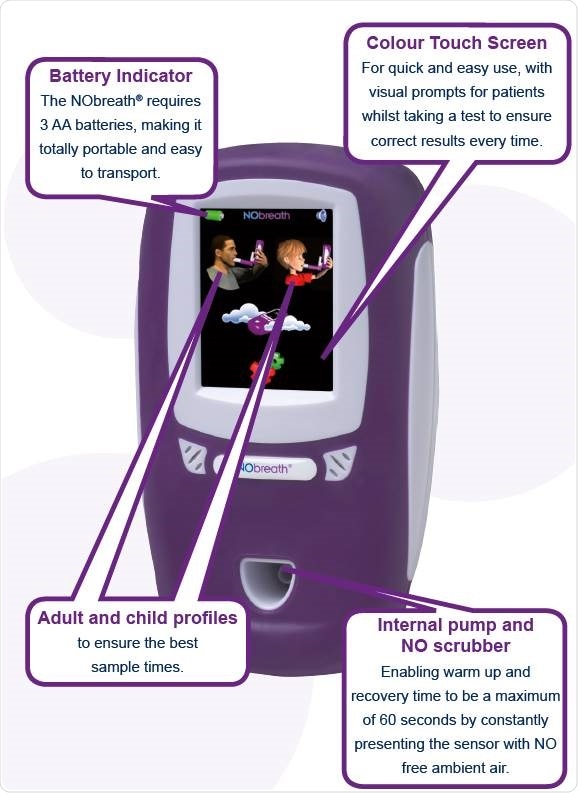
Image credit: Bedfont Scientific
Measuring FeNO with NObreath® is as easy as 1, 2, 3

Inhale. Image credit: Bedfont Scientific

Exhale. Image credit: Bedfont Scientific

Readings instantly available. Image credit: Bedfont Scientific
Technical specification
|
Concentration range
|
5-300ppb nitric oxide
|
|
Accuracy
|
± 5ppb of measured value ≤50ppb
± 10% of measured value >50ppb
|
|
Repeatability
|
± 5ppb of measured value ≤50ppb
± 10% of measured value >50ppb
|
|
Sensor sensitivity
|
5ppb
|
|
Breath test time
|
Adult 12 seconds
Child 10 seconds
|
|
Response time
|
<10 secs
|
|
Warm up time
|
~60 secs
|
|
Ambient air test
|
30 seconds
|
|
Operating temperature range
|
10-30 ºC (ambient)
|
|
Operating relative humidity (environmental)
|
10-80% Rh (non-condensing)
|
|
Sensor operating life
|
1-2 years; 6 month warranty
|
|
Detection principle
|
Electrochemical sensor
|
|
Sensitivity drift
|
<5% per annum
|
|
Maximum ambient operating level
|
350 ppb NO
|
|
Power
|
4.5V DC: 3 x AA (LR6 or equivalent) alkaline batteries
|
|
Battery life (1 set of 3 AA batteries)
|
Up to 120 tests
|
|
Display
|
Colour LCD with touch screen
|
|
Dimensions
|
Approx. 152 x 87 x 47mm
|
|
Weight
|
Approx. 400g including batteries
|
|
Construction
|
Case: polycarbonate/ABS blend with elastomeric overmould NObreathFlo™: polycarbonate/ABS blend Mouthpiece: polypropylene
|
References
- Shelhamer JH, Levine SJ, Wu T, Jacoby DB, Kaliner MA, Rennard SI. NIH conference: airway inflammation. Ann Intern Med 1995;123:288-304.
- ATS/ERS Recommendations for Standardized Procedures for the Online and Offline Measurement of Exhaled Lower Respiratory Nitric Oxide and Nasal Nitric Oxide, 2005; American Journal of Respiratory and Critical Care Medicine; vol. 171: 912-930;2005
- Andrew D. Smith, Jan O. Cowan, Sue Filsell, Chris MacLachlan, Gabrielle Monti-Sheehan, Pamela Jackson and D. Robin Taylor. Diagnosing Asthma: Comparisons between Exhaled Nitric Oxide Measurements and Conventional Tests. Am J Respir Crit Care Med Vol 169. pp 473-478, 2004.
- Coumou HBel E. Improving the diagnosis of eosinophilic asthma [Internet]. Taylor and Francis online. 2017 [cited 15 March 2017]. Available from: http://www.tandfonline.com/doi/full/10.1080/17476348.2017.1236688
- D R Taylor, MW Pinenburg, A D Smith and J C D Jongste. Exhaled nitric oxide measurements: clinical application and interpretation. Thorax 2006;61:817-827.
- Saito J, Gibeon D, Macedo P, Menzies-Gow A, Bhavsar P, Chung K. Domiciliary diurnal variation of exhaled nitric oxide fraction for asthma control. 2017.
- Correlation of Exhaled Nitric Oxide, Spirometry and Asthma Symptoms: Journal of Asthma: Vol 42, No 10 [Internet]. Tandfonline.com. 2017 [cited 15 March 2017]. Available from: http://www.tandfonline.com/doi/abs/10.1080/02770900500371344
About Bedfont Scientific
Bedfont® Scientific has specialised in the design and manufacture of exhaled breath and gas monitoring instruments since 1976.
For medical gas monitoring, their Medi-Gas Check medical pipeline testing range verifies not only the quantity but also quality of gas administered to patients.
Bedfont's breath analysers include carbon monoxide (CO) monitors such as the Smokerlyzer®, used for smoking cessation, and the ToxCO®, used by emergency services, to diagnose CO poisoning.
The NObreath® FeNO monitor provides accurate analysis of airway inflammation for the control of asthma, and the Gastrolyzer® range aids in the detection of gastrointestinal disorders and food intolerances. Quick and non-invasive, breath analysis is the new blood test.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.