2. What should I know before I use FEIBA NF?
Do not use if you are allergic to FEIBA NF or any of the ingredients listed at the
end of the CMI. Do not use if you are suffering from a blood clotting condition called
Disseminated Intravascular Coagulation. Talk to your doctor if you have or have had
any other medical conditions, if you take any other medicines, if you are pregnant
or plan to become pregnant or if you are breastfeeding or plan to breastfeed. If you
are taking a medicine called emicizumab, it is very important you talk to your doctor
before using FEIBA NF. For more information, see Section
2. What should I know before I use FEIBA NF? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with FEIBA NF and how it works. Tell your doctor or Haemophilia
Treatment Centre if you are taking or using any other medicines including any that
you get without a prescription from your pharmacy, supermarket, or health food shop.
For more information, see Section
3. What if I am taking other medicines? in the full CMI.
4. How will I be given FEIBA NF?
FEIBA NF injection is prepared and administered by a qualified healthcare professional
who is experienced in the care of patients with haemophilia.
Your dose of FEIBA NF is dependent on your condition and body weight, and your dose
may change during treatment.
The frequency of your infusions will depend on how well FEIBA NF is working for you.
FEIBA NF is given slowly by injection directly into your veins.
FEIBA NF is supplied as a dry powder and the powder must be dissolved with the diluent
supplied in the pack before use.
5. What should I know while using FEIBA NF?
|
Things you should do
|
Tell your doctor or Haemophilia Treatment Centre immediately if you notice any sudden
signs and symptoms of a severe, sudden allergic reaction.
If you are on emicizumab before or while using FEIBA NF, seek urgent medical attention
if you notice any symptoms of thrombotic microangiopathy or blood clots.
Tell your doctor or Haemophilia Treatment Centre immediately if FEIBA NF is no longer
helping your condition (e.g., if you noticed an increase in bleeds).
Tell any doctors, dentists, or pharmacists you visit that you are using FEIBA NF.
Keep all your appointments with your doctor and any blood tests.
|
|
Things you should not do
|
Do not give your medicine to anyone else, even if they appear to have the same condition
as you.
Do not stop using your medicine or change the dosage without checking with your doctor.
|
|
Looking after your medicine
|
Store FEIBA NF below 25°C.
Keep FEIBA NF in the pack until it is time to use it so that it is protected from
light.
|
6. Are there any side effects?
Common side effects include headache, dizziness, light-headedness, rash, allergies,
hepatitis B surface antibody positive. Serious side effects include a condition called
disseminated intravascular coagulation, blood clots in the veins or lungs, heart attack,
stroke, severe sudden allergic reaction which may progress to difficulty in breathing,
chest pain and fainting. For more information, including what to do if you have any
side effects, see Section
6. Are there any side effects? in the full CMI.
Active ingredient(s):
factor VIII inhibitor bypassing fraction
Full Consumer Medicine Information (CMI)
This leaflet provides important information about using FEIBA NF.
You should also speak to your doctor or pharmacist if you would like further information
or if you have any concerns or questions about using FEIBA NF.
Where to find information in this leaflet:
1. Why am I using FEIBA NF?
FEIBA NF contains the active ingredient factor VIII inhibitor bypassing fraction (a
human, plasma-derived protein with factor VIII inhibitor bypassing activity expressed).
FEIBA NF is used to help clotting in patients with haemophilia A or B who have developed
inhibitors (antibodies) against the blood clotting factor VIII and factor IX, respectively.
FEIBA NF is used:
to treat bleeding episodes in haemophilia A or B patients with inhibitors,
to prevent bleeding episodes in haemophilia A or B patients with inhibitors,
in haemophilia A or B patients with inhibitors to prevent or reduce bleeding before,
during and after surgery.
The blood clotting factor VIII and factor IX are essential for the blood to form clots
and stop bleedings. Patients with haemophilia A have lower factor VIII levels than
normal. Patients with haemophilia B have lower factor IX levels than normal. During
the course of treatment with replacement therapy, some haemophilia A and haemophilia
B patients can develop antibodies against factor VIII and factor IX, respectively.
This results in the replacement therapy from working properly and blood will not clot
properly.
FEIBA NF is a concentrate of blood factors normally present in your blood which can
bypass the effects of these antibodies to make sure that your blood clots properly.
This medicine helps to control your condition but does not cure it.
Ask your doctor if you have any questions about why this medicine has been prescribed
for you.
Your doctor may have prescribed it for another reason.
2. What should I know before I use FEIBA NF?
Warnings
Do not use FEIBA NF if:
you are allergic to factor VIII inhibitor bypassing fraction, or any of the ingredients
listed at the end of this leaflet. Always check the ingredients to make sure you can
use this medicine.
you are suffering from Disseminated Intravascular Coagulation (a blood clotting condition
that can cause blood clots, bleeding and sudden bruising). This condition usually
occurs after a serious disease, injury, or after a major operation, diagnosed by a
blood test.
Check with your doctor if:
you have suffered from a heart attack.
you have a blood clot.
you have liver problems.
your immune system is not working properly.
you have or have had any other medical conditions.
During treatment, you may be at risk of developing certain side effects. It is important
you understand these risks and how to monitor for them. See additional information
under Section
6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant. There is
no information on the use of FEIBA NF during pregnancy. Your doctor will discuss the
risks and benefits of using FEIBA NF if you are pregnant.
Talk to your doctor if you are breastfeeding or intend to breastfeed. It is not known
if FEIBA NF passes into your milk and if it can harm your baby. Your doctor will discuss
the risks and benefits of using FEIBA NF if you are breast-feeding.
3. What if I am taking other medicines?
Tell your doctor or Haemophilia Treatment Centre if you are taking any other medicines,
including any medicines, vitamins or supplements that you buy without a prescription
from your pharmacy, supermarket or health food shop.
Some medicines may interfere with FEIBA NF and affect how it works. These include:
anti-fibrinolytics (medicines which help make blood clots more stable and last longer),
for example aminocarproic acid, tranexamic acid, recombinant activated factor VII.
emicizumab (a medicine used to prevent bleeding in haemophilia A patients).
If you are taking any of the above medicines while receiving FEIBA NF, be aware of
the possibility of serious side effects. Your doctor will need to monitor you closely.
Your doctor or Haemophilia Treatment Centre have more information on medicines to
be careful with or avoid if you have concerns.
4. How do I use FEIBA NF?
Treatment with FEIBA NF should be started in a hospital or Haemophilia Treatment Centre
and supervised by your doctor who is experienced in the care of patients with haemophilia.
Each time you use FEIBA NF, record the name and batch number of the medicine.
How much to use
Follow all directions given to you by your doctor carefully. They may differ from
the information contained in this leaflet.
Your doctor will decide how much FEIBA NF you use, how often and at what intervals
you need to have it.
Your dose will depend on:
your body weight,
how much, how often and where you are bleeding.
A dose of 50 to 100 Units per kilogram of body weight (U/kg) is recommended.
The maximum single dose should not be more than 100 U/kg.
The maximum daily dose should not be more than 200 U/kg.
How to use FEIBA NF
FEIBA NF is given by slow injection or infusion directly into your vein.
Some individuals may be trained to use FEIBA NF at home.
Do not attempt to inject FEIBA NF by yourself unless you have received proper training
by your doctor or Haemophilia Treatment Centre on how to use the product.
Preparing FEIBA NF
FEIBA NF is supplied as a powder and must be mixed with water for injections (diluent
supplied in the pack) to form a clear solution before use.
Follow carefully the step-by-step instructions at the end of this leaflet or in the
pack insert on how to prepare and inject FEIBA NF.
Do not mix FEIBA NF with any other medicines or solvent other than the water for injections
diluent supplied with the pack.
Use only the reconstitution device provided with each pack to prepare the solution
for injection.
After mixing the powder and the diluent, use the solution immediately. If the solution
is not used straight way, you can keep the solution for a maximum of 3 hours when
stored at room temperature.
Do not refrigerate the solution after it is prepared.
FEIBA NF is for single use in one patient only.
Dispose of all unused solution, empty vials, and used needles and syringes into a
sharps bin.
If you are unsure about how to prepare the medicine for use, contact your doctor or
Haemophilia Treatment Centre.
Inspecting FEIBA NF
Always inspect FEIBA NF before use and after it has been mixed.
After mixing, the solution should be clear to colourless, and free from foreign particles.
Do not inject the solution if it is discoloured, or cloudy, or contains particles.
How often to use FEIBA NF
Your doctor will tell you how often or at what intervals you will receive the injection.
The frequency of injections you receive, and how long you will use FEIBA NF for, will
depend on how well FEIBA NF is working for you.
Continue using FEIBA NF for as long as your doctor tells you. Usually, the replacement
therapy with FEIBA NF is a life-long treatment.
If you forget to use FEIBA NF
If you forget your scheduled injection, do not inject a double dose to make up for
the dose your missed.
If you inject a double dose, this may increase the chance of you getting an unwanted
side effect.
If you are not sure what to do, ask your doctor or Haemophilia Treatment Centre.
If you use too much FEIBA NF
If you think that you have used too much FEIBA NF, you may need urgent medical attention.
You should immediately:
contact your doctor or Haemophilia Treatment Centre, or
go to the Emergency Department at your nearest hospital, or
phone the Poisons Information Centre by calling
13 1126 (if you are in Australia), or by calling 0800 764 766 (if you are in New Zealand).
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using FEIBA NF?
Things you should do
Tell you other doctors, dentists and pharmacists you are using this medicine.
If you are about to have any blood tests, tell your doctor that you are using FEIBA
NF.
Keep all your doctor's appointments so that your progress can be checked. Your doctor
may do some blood tests before you start your treatment and from time to time during
your treatment to monitor your progress.
Follow all instructions from your doctor. Your doctor may change the dose you use
using your treatment.
Tell your doctor and/or Haemophilia Treatment Centre straight away if you notice:
any sudden signs and symptoms of a severe allergic response.
your bleeding is not controlled or worsens after using FEIBA NF.
Things you should not do:
Do not give your medicine to anyone else, even if they appear to have the same condition
as you.
Do not use FEIBA NF to treat any other complaints unless your doctor tells you to.
Do not stop using FEIBA NF unless advised by your doctor or healthcare professional
or unless you have an allergic reaction.
Do not change the dosage without checking with your doctor.
Do not use FEIBA NF after the expiry date which is printed on the label after the
word 'EXP'. The expiry date refers to the last day of the month.
Driving or using machines
FEIBA NF is not expected to have an influence on your ability to drive and use machines.
Be careful before you drive or use any machines or tools until you know how FEIBA
NF affects you.
Looking after your medicine
Keep FEIBA NF in the pack until it is time to use it. This will protect the medicine
from light.
Store FEIBA NF below 25°C.
Follow the instructions in the carton on how to take care of your medicine properly.
Keep FEIBA NF out of reach of children.
Getting rid of any unwanted medicine
Medicines should not be disposed of via wastewater or household waste.
If your doctor tells you to stop using this medicine, or if the medicine is out of
date, or if the medicine has not been stored properly, ask your doctor or Haemophilia
Treatment Centre what to do with any unwanted medicine that is left over.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of
them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you
have any further questions about side effects.
Less serious side effects
Serious side effects
Signs or symptoms of a severe sudden allergic response include:
rash, hives, wheals, or generalised itching
swelling of face, lips and tongue which may cause difficulty in swallowing,
shortness of breath, wheezing, difficulty in breathing,
tightness or discomfort in the chest, dizziness and fainting
Your body can make antibodies (also called "inhibitors") against FEIBA NF. If this
happens, FEIBA NF may stop working properly, signs or symptoms include easily bruising
or bleeding, swelling and pain or tightness in joints. This is a very common side
effect in patients who have not been previously treated with factor VIII medicines.
In patients who have not been previously treated with factor VIII medicines, this
side effect is uncommon.
Tell your doctor, Haemophilia Treatment Centre, or pharmacist if you notice anything
else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
By reporting side effects, you can help provide more information on the safety of
this medicine.
Always make sure you speak to your doctor, Haemophilia Treatment Centre, or pharmacist
before you decide to stop using any of your medicines.
7. Product details
What FEIBA NF contains
Powder in a vial
|
Active ingredient
(main ingredient)
|
Factor VIII inhibitor bypassing fraction*
|
|
Other ingredients
(inactive ingredients)
|
sodium chloride
sodium citrate dihydrate
|
* FEIBA NF also contains factors II, IX and X, mainly in non-activated form as well
as activated factor VII. Factor VIII coagulant antigen (FVIII C:Ag) and the factors
of the kallikrein-kinin system are present only in trace amounts, if at all.
Diluent in a vial
|
Other ingredients
(inactive ingredients)
|
water for injections
|
Do not take this medicine if you are allergic to any of these ingredients.
What FEIBA NF looks like
FEIBA NF is a white to cream powder supplied in a single-dose glass vial.
Each pack of FEIBA NF contains:
1 drug powder vial containing 500 U, 1000 U or 2500 U of FEIBA NF
1 diluent vial containing 10 mL, 20 mL or 50 mL of water for injections
1 BAXJECT II Hi-Flow reconstitution device
FEIBA NF is available in the following strengths:
FEIBA NF 500 U - AUST R 104896
FEIBA NF 1000 U - AUST R 104911
FEIBA NF 2500 U - AUST R 172236
Not all presentations may be marketed.
Who distributes FEIBA NF
FEIBA NF is supplied in Australia by:
Takeda Pharmaceuticals Australia Pty Ltd
Level 39, 225 George Street
Sydney NSW 2000
Australia
Telephone: 1800 012 612
www.takeda.com/en-au
FEIBA NF is supplied in New Zealand by:
Takeda New Zealand Pty Limited
Level 10, 21 Queen Street
Auckland 1010
New Zealand
Telephone: 0508 169 077
www.takeda.com/en-au
This leaflet was prepared in January 2024.
8. Instructions for use
IMPORTANT
Contact your doctor or Haemophilia Treatment Centre if you have any questions or if
you experience any problems following this instruction guide.
These instructions are intended only as a visual aid for those patients who have been
trained by their doctor or Haemophilia Treatment Centre on the proper way to self-inject
the medicine.
Do not attempt to inject FEIBA NF by yourself unless you have been trained by your
doctor or Haemophilia Treatment Centre on how to use the product.
Use only the water for injections (diluent) and the reconstitution device provided
in the pack to prepare the solution for injection.
If more than one vial of FEIBA NF is needed for the dose, mix each vial of FEIBA NF
using a separate reconstitution device supplied in each pack.
Follow the step-by-step instructions specific to the reconstitution device supplied
with your FEIBA NF.
After preparing FEIBA NF, use the solution as soon as possible, within 3 hours after
mixing.
Always inspect FEIBA NF before use and after it has been mixed. After mixing, the
solution should be clear to colourless.
Do not use the solution if it is discoloured, or cloudy, or contains particles.
Preparing FEIBA NF using aseptic technique
In a quiet place, prepare a clean surface and gather all the materials you will need
for the injection.
Remove FEIBA NF from the refrigerator and check the expiry date on the package.
Wash your hands and put on clean exam gloves. If you are self-injecting at home the
use of gloves is optional.
Using the BAXJECT II device
Use aseptic technique (clean and germ free) and a flat work surface during the reconstitution
procedure.
1. Allow the FEIBA NF powder and diluent vials to reach room temperature before use.
2. Remove plastic caps from the FEIBA NF powder and diluent vials.
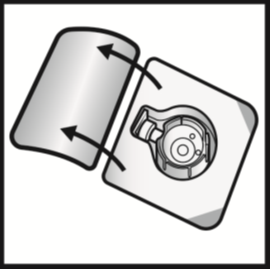
3. Cleanse rubber stoppers with an alcohol wipe and allow to dry before use.Open the BAXJECT II device package by peeling away the lid, without touching the inside
(Figure A). Do not remove the device from the package.
Figure A
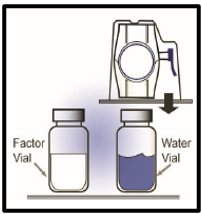
4. Turn the package over. Press straight down to fully insert the clear plastic spike
through the diluent vial stopper (Figure B).
Figure B
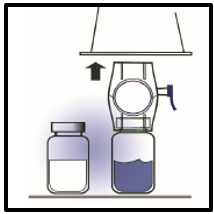
5. Grip the BAXJECT II package at its edge and pull the package off the device (Figure
C). Do not remove the blue cap from the BAXJECT II device. Do not touch the exposed
white plastic spike.
Figure C
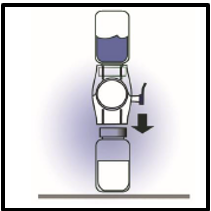
6. Turn the system over so that the diluent vial is on top. Quickly insert the white
plastic spike fully into the FEIBA NF powder vial stopper by pushing straight down
(Figure D). The vacuum will draw the diluent into the FEIBA NF powder vial.
Figure D
7. Swirl gently until the FEIBA NF powder is completely dissolved. DO NOT SHAKE.
Administration
8. Remove the blue cap from the BAXJECT II device. Connect the syringe to the BAXJECT
II (Figure E). DO NOT INJECT AIR into the BAXJECT II device.
Figure E
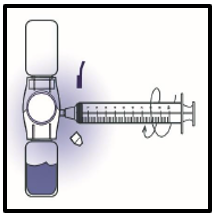
9. Turn the system upside down (FEIBA NF powder vial now on top). Draw the reconstituted
solution into the syringe by pulling the plunger back slowly (Figure F).
Figure F
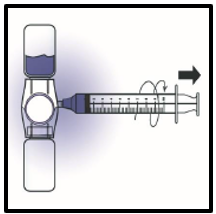
10. Disconnect the syringe; attached a suitable needle and inject slowly into the vein
over a period of up to 5 minutes (maximum infusion rate of 10 mL per minute).
11. If more than one vial of FEIBA NF is to be used, the contents of multiple vials may
be drawn into the same syringe.
FEIBA NF® and BAXJECT® are registered trademarks of Baxalta Incorporated.
TAKEDA and the TAKEDA Logo are registered trademarks of Takeda Pharmaceutical Company
Limited.