2. What should I know before I use LUVERIS?
Do not use if you have ever had an allergic reaction to LUVERIS or any of the ingredients
listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines,
are pregnant or are breastfeeding.
3. What if I am taking other medicines?
Some medicines may interfere with LUVERIS and affect how it works.
4. How do I use LUVERIS?
It is usually recommended that your treatment with LUVERIS starts at 75 IU daily along
with 75 IU or 150 IU of follitropin alfa. Your doctor may adjust your dose depending
on your individual response to treatment.
LUVERIS is given as a course of daily subcutaneous (under the skin) injection at the
same time as another medicine, follitropin alfa.
Follow all directions given to you by your doctor or pharmacist carefully, including
the Instructions for Use provided in the pack.
5. What should I know while using LUVERIS?
|
Things you should do
|
Remind any doctor, dentist or pharmacist you visit that you are using LUVERIS.
Tell your doctor if you become pregnant while using LUVERIS.
|
|
Things you should not do
|
Do not stop using this medicine suddenly without telling your doctor.
Do not change the dose unless your doctor tells you to.
Do not give this medicine to anyone else, even if their symptoms seem similar to yours.
|
|
Driving or using machines
|
Be careful driving or operating machinery until you know how LUVERIS affects you.
|
|
Looking after your medicine
|
Prior to reconstitution keep the vials in the original package in a dry cool place
where the temperature is below 25˚C.
Once the LUVERIS powder is dissolved with the solvent provided, it should be injected
immediately. Any solution left over must be discarded.
|
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of
them are minor and temporary. However, some side effects may need medical attention.
Most common side effects include injection site reactions, headache, nausea, vomiting,
diarrhoea, abdominal pain or discomfort. Tell your doctor if you experience any side
effects.
Active ingredient:
lutropin alfa (rch)
Full Consumer Medicine Information (CMI)
This leaflet provides important information about using LUVERIS. You should also speak to your doctor or pharmacist if you would like further information
or if you have any concerns or questions about using LUVERIS.
Where to find information in this leaflet:
1. Why am I using LUVERIS?
LUVERIS contains the active ingredient lutropin alfa (rch), a recombinant luteinising
hormone (LH). This hormone is essentially similar to the hormone found naturally in human, but
it is made by means of biotechnology. LUVERIS belongs to the family of hormones called
gonadotrophins, which are involved in the normal control of reproduction.
LUVERIS is used for the stimulation of follicular development in women with severe
LH and FSH deficiency.
2. What should I know before I use LUVERIS?
Warnings
Do not use LUVERIS if:
you are allergic to lutropin alfa (rch), or any of the ingredients listed at the end
of this leaflet.
Always check the ingredients to make sure you can use this medicine.
you are pregnant or breastfeeding
you have an unexplained ovarian cyst or ovarian enlargement
you have unexplained vaginal or uterine bleeding
you have cancer of the ovaries, uterus or breasts
you have tumours of the pituitary gland or hypothalamus
Check with your doctor if you:
take any medicines for any other condition
have or have had any other medical conditions, such as:
kidney disease
liver disease
you or your family have increased risk factors for developing blood clots, e.g. stroke,
heart attacks
porphyria or a family history of porphyria
During treatment, you may be at risk of developing certain side effects. It is important
you understand these risks and how to monitor for them. See additional information
under Section
6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant while using LUVERIS.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
Compared to natural conception, the frequency of multiple pregnancies and births is
increased in patients receiving this treatment. Your doctor will monitor your ovarian
response to minimise the chance of multiple pregnancies.
There may be a slightly increased risk of birth defects in women using assisted reproductive
technologies. This may be due to increased maternal age, genetic factors, multiple
pregnancies or the procedures.
Talk to your doctor about any concerns you may have before undergoing treatment with
LUVERIS.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any
medicines, vitamins or supplements that you buy without a prescription from your pharmacy,
supermarket or health food shop.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins
or supplements you are taking and if these affect LUVERIS.
4. How do I use LUVERIS?
How much to use
It is recommended that your treatment with LUVERIS starts with 75 IU daily, together
with 75 IU to 150 IU of follitropin alfa.
Your treatment should be tailored according to your individual response. Your doctor
will tell you how much LUVERIS to use.
When to use LUVERIS
Your doctor will tell you when to take LUVERIS.
LUVERIS is given as a course of daily injections. You should have your injection at
the same time each day.
How to use LUVERIS
LUVERIS should be injected daily under the skin (subcutaneous) in the lower abdominal
area or thigh.
Each vial is for single use only.
The injection site should be changed daily to lessen possible injection site reactions.
Before using LUVERIS, your doctor or nurse can teach you the injection technique.
Read the Instructions for Use carefully, as follows:
It is important that your hands and the items you use be as clean as possible.
2.
Assemble everything you need
Please note that alcohol swabs, syringes and needles are not provided in the package.
Find a clean area and lay out everything:
Two alcohol swabs
One solvent vial
One vial containing LUVERIS
One syringe
One big needle for reconstitution
A fine-bore needle for subcutaneous injection
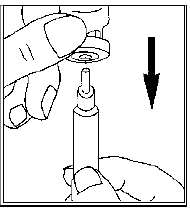
Remove the protective cap from the solvent vial. Attach the needle for reconstitution
(the bigger needle) to the syringe and draw up some air into the syringe by pulling
the plunger to approximately the 1 mL mark. Then insert the needle into the vial,
push the plunger to expel the air, turn the vial upside down and gently draw up all
the solvent.
4.
Prepare the injection solution
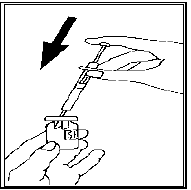
Remove the protective cap from LUVERIS powder vial, slowly inject the solvent in the
syringe into the vial of LUVERIS.
Swirl gently without removing the syringe. Do not shake.
The powder will dissolve into a clear solution immediately. Do not use the solution
if it is not clear.
Turn the vial upside down, gently draw the solution back into the syringe
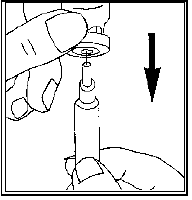
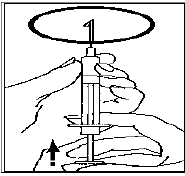
Change the needle for the fine-bore needle and remove any air bubbles: If you see
air bubbles in the syringe, hold the syringe with the needle pointing upwards and
gently flick the syringe until all the air collects at the top.
Gently push the plunger until the air bubbles are gone.
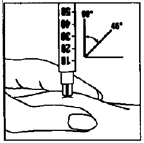
Immediately inject the solution. Your doctor or nurse will tell you where to inject
(e.g. tummy, front of thigh).
Wipe the chosen area with an alcohol swab. Firmly pinch the skin together and insert
the needle at a 45° to 90° angle using a dart-like motion. Inject under the skin,
as you were taught. Do not inject directly into a vein.
Inject the solution by pushing gently on the plunger. Take as much time as you need
to inject all the solution.
Immediately withdraw the needle and clean the skin with an alcohol swab or cotton
pad.
6.
Dispose of all used items
Once you have finished your injection, immediately discard all needles and empty glass
containers in a sharps container. Any unused solution must be discarded.
If you forget to use LUVERIS
LUVERIS should be used regularly at the same time each day. If you miss your dose
at the usual time, contact your doctor or nurse immediately for advice.
If you use too much LUVERIS
If you think that you have used too much LUVERIS, you may need urgent medical attention.
You should immediately:
phone the Poisons Information Centre
(by calling
13 11 26), or
contact your doctor, or
go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using LUVERIS?
Things you should do
See your doctor regularly. Your doctor will monitor you closely throughout your treatment.
Tell your doctor immediately if you become pregnant while using LUVERIS.
If you plan to have surgery, tell your doctor or dentist that you are using LUVERIS.
Remind any doctor, dentist or pharmacist you visit that you are using LUVERIS.
Things you should not do
Do not stop using this medicine suddenly without telling your doctor.
Do not change the dose unless your doctor tells you to.
Do not give this medicine to anyone else even if they have the same condition as you.
Ovarian Hyperstimulation Syndrome (OHSS)
Treatment with LUVERIS may increase your risk of developing a condition called ovarian
hyperstimulation syndrome (OHSS). This is when the ovaries overact to the hormonal
treatment and become larger.
The most common symptom is lower abdominal pain. Your doctor will monitor your treatment
using ultrasound and blood tests to help determine if you are likely to develop OHSS.
If necessary, your doctor will delay or cancel your LUVERIS injection. You may also
be advised to refrain from sexual intercourse or use barrier methods until the end
of the cycle if this occurs.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how LUVERIS
affects you.
Looking after your medicine
Prior to reconstitution keep the vials in a dry cool place where the temperature is
below 25˚C.
Store in the original package in order to protect from light.
Once the LUVERIS powder is dissolved with the solvent provided, it should be injected
immediately. This is due to the solvent not containing any preservative. Any solution
that is left over must be discarded.
Do not use the dissolved solution if it contains particles or is not clear.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do
not store it:
in the bathroom or near a sink, or
in the car or on window sills.
Keep it where young children cannot reach it.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy
for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of
them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you
have any further questions about side effects.
Side effects
|
Side effects
|
What to do
|
|
Injection site reactions, such as bruising, pain, redness, itching or swelling
Headache
Nausea, vomiting, diarrhoea
Abdominal pain or discomfort
|
Speak to your doctor if you have any of these side effects and they worry you.
|
Serious side effects
|
Serious side effects
|
What to do
|
|
Allergic reactions (very rare)
Severe skin rash, itching or hives
Swelling of the face, lips, tongue or other parts of the body
Shortness of breath, wheezing or difficulty breathing
Ovarian Hyperstimulation Syndrome (OHSS):
Signs of OHSS:
Lower abdominal pain, discomfort or swelling
Nausea, vomiting, diarrhoea
Pelvic pain
Very rarely, blood cloth (thromboembolism) associated with severe OHSS may occur.
Signs of blood cloth include pain, warmth, redness, numbness or tingling in arm or
legs, warning signs of stroke or heart attack.
|
Call your doctor straight away, or go straight to the Emergency Department at your
nearest hospital if you notice any of these serious side effects.
|
Tell your doctor or pharmacist if you notice anything else that may be making you
feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
Always make sure you speak to your doctor or pharmacist before you decide to stop
taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What LUVERIS contains
|
Active ingredient
(main ingredient)
|
Lutropin alfa (rch)
|
|
Other ingredients
(inactive ingredients)
|
Polysorbate 20
Dibasic sodium phosphate dihydrate
Monobasic sodium phosphate monohydrate
Methionine
Sucrose
Phosphoric acid
Sodium hydroxide
|
Do not take this medicine if you are allergic to any of these ingredients.
What LUVERIS looks like
LUVERIS is a sterile white powder in a vial.
Each vial of LUVERIS contains 75 IU lutropin alfa.
LUVERIS is available in packs of 1 vial. Each pack also contains a vial of 1 mL water
for injections.
Australian Registration Number: AUST R 95042
Who distributes LUVERIS
Luveris is supplied in Australia by:
Merck Healthcare Pty Ltd
Suite 1, Level 1, Building B
11 Talavera Road
Macquarie Park NSW 2113
For enquiries call: 1800 633 463
Luveris is supplied in New Zealand by:
Healthcare Logistics
58 Richard Pearse Drive
Airport Oaks, Auckland
For enquiries call: 0800 426 252
This leaflet was prepared in June 2024.