2. What should I know before I use NIVESTIM?
Do not use if you have ever had an allergic reaction to filgrastim or any of the ingredients
listed at the end of the CMI, or any other products that are produced using the bacteria
E. coli. Do not use NIVESTIM 24 hours before or after you receive your chemotherapy,
radiotherapy, bone marrow transplant or stem cell transplant.
Talk to your doctor if you have any other medical conditions, take any other medicines,
or are pregnant or plan to become pregnant or are breastfeeding. For more information, see Section
2. What should I know before I use NIVESTIM? in the full CMI.
3. What if I am taking other medicines?
4. How do I use NIVESTIM?
NIVESTIM is given by injection, usually just below the skin (called a subcutaneous
injection) and it is a simple procedure. More instructions can be found in Section
4. How do I use NIVESTIM? in the full CMI.
5. What should I know while using NIVESTIM?
|
Things you should do
|
Remind any doctor, dentist, pharmacist or nurse you visit that you are using NIVESTIM.
Keep all your doctor’s appointments so that your health can be monitored.
Call your doctor straight away if you become pregnant.
Go straight to the hospital if you notice any signs or symptoms of infection.
|
|
Things you should not do
|
Do not stop using this medicine or lower the dosage without checking with your doctor.
Do not use NIVESTIM to treat any other complaint unless your doctor tells you to.
|
|
Driving or using machines
|
Be careful driving or using any machines or tools until you know how NIVESTIM affects
you.
|
|
Looking after your medicine
|
Keep NIVESTIM in a refrigerator at a temperature of 2°C to 8°C.
Keep your medicine in its pack until it is time to use it. Protect it from light.
|
6. Are there any side effects?
Side effects may include bone, back, muscle, joint, mouth or throat pain; swelling
or stiffness of joints; muscle spasms; abdominal discomfort; diarrhoea; constipation;
nausea; vomiting; severe nose bleeds; reddish or purplish bumps or blotches; mouth
ulcers; numbness, tingling in the hands and feet; injection site reactions; cough;
hair loss; headache; looking pale; loss of appetite; unusual weakness; difficulty
sleeping. Serious side effects include rash, itching or hives; swelling of the face,
lips, mouth or throat; difficulty swallowing or breathing; shortness of breath; wheezing;
light-headedness, dizziness or fainting; rapid pulse or breathing, sweating; painful
skin lesions; swelling; chest, abdominal or back pain; fever; tiredness; easy bruising
or bleeding; left shoulder pain; frequent infections; coughing up blood or mucus;
pain in the upper left side of the stomach; swelling of your stomach-area; reduced
urination; blood in the urine. For more information, including what to do if you have
any side effects, see Section
6. Are there any side effects? in the full CMI.
Active ingredient(s):
Filgrastim [rbe]
Full Consumer Medicine Information (CMI)
This leaflet provides important information about using NIVESTIM. You should also speak to your doctor or pharmacist if you would like further information
or if you have any concerns or questions about using NIVESTIM.
Where to find information in this leaflet:
1. Why am I using NIVESTIM?
NIVESTIM contains the active ingredient filgrastim (rbe). NIVESTIM belongs to a group of medicines called cytokines and is a copy of a substance
normally present in your body, called Granulocyte Colony Stimulating Factor or G-CSF.
Using gene technology, NIVESTIM is produced in a specific type of bacteria, called
E. coli.
G-CSF is produced in the bone marrow and assists in the production of neutrophils,
which are a type of white blood cell. Neutrophils help the body fight infections by
surrounding and destroying the bacteria that cause them. G-CSF also helps neutrophils
to do this work better. NIVESTIM works by encouraging the bone marrow to produce more
neutrophils.
NIVESTIM can be used:
to increase the number of white blood cells after treatment with chemotherapy to help
prevent infections;
to increase the number of white blood cells after a bone marrow transplant to help
prevent infections;
before high-dose chemotherapy to make the bone marrow produce more stem cells which
can be collected and given back to you after your treatment. These can be taken from
you or from a donor. The stem cells will then go back into the bone marrow and produce
blood cells;
to increase the number of white blood cells if you suffer from severe chronic neutropenia
to help prevent infections;
in patients with advanced HIV infection which will help reduce the risk of infections.
2. What should I know before I use NIVESTIM?
Warnings
Do not use NIVESTIM:
if you are allergic to filgrastim, any of the ingredients listed at the end of this
leaflet, or any medicines or products that are produced using the bacteria E. coli.
Some of the symptoms of an allergic reaction may include shortness of breath, wheezing
or difficulty breathing, swelling of the face, lips, tongue or other parts of the
body, rash, itching or hives on the skin.
Always check the ingredients to make sure you can use this medicine.
in the 24 hours before or after you receive your chemotherapy, radiotherapy, bone
marrow transplant or stem cell transplant.
This is because these types of treatments may stop NIVESTIM from increasing the number
of infection-fighting neutrophils.
Check with your doctor if you:
have any allergies to any other medicines, foods, preservatives or dyes
have a medical condition affecting the bone marrow or blood
have a family history of a genetic disorder
suffer from sickle cell disease (an inherited disease in which red blood cells are
sickle shaped)
have problems with your kidneys, liver, heart or other organs such as past problems
with your spleen e.g. splenomegaly (enlarged spleen)
have had previous treatment for cancer
have any infections, cancers or tumours
have a recent history of pneumonia (or other serious lung infections)
have osteoporosis (weakening of the bones) or other bone diseases
have HFI (hereditary fructose intolerance) - NIVESTIM contains sorbitol which may
affect this condition
take any medicines for any other condition.
During treatment, you may be at risk of developing certain side effects. It is important
you understand these risks and how to monitor for them. See additional information
under Section
6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
Your doctor can discuss with you the risks and benefits involved.
Use in Children
There is limited experience with the use of NIVESTIM in children. Your doctor will
discuss the risks and benefits of using it in children.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, particularly
those that may affect the blood. Also tell him/her about any medicines, vitamins or
supplements that you buy without a prescription from your pharmacy, supermarket or
health food shop.
Some medicines may interfere with NIVESTIM and affect how it works.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins
or supplements you are taking and if these affect NIVESTIM.
4. How do I use NIVESTIM?
How much NIVESTIM to use
Your doctor will tell you the strength of NIVESTIM you need and how much you require.
How much you need will depend on the reason for your treatment, your body weight and
the number of neutrophils in your blood.
For NIVESTIM to work properly, you have to use it exactly as your doctor has instructed.
Follow all directions given to you by your doctor, pharmacist or nurse carefully.
When to use NIVESTIM
For the best effect you should inject NIVESTIM at about the same time each day. Your
doctor will tell you when to begin your treatment and when to stop.
How to use NIVESTIM
NIVESTIM is given by injection, usually into the tissues just below the skin. This
is called a subcutaneous injection and it is a simple procedure.
Your doctor, nurse or pharmacist may suggest that you or your carer be taught how
to give a subcutaneous injection. This will allow you to have your NIVESTIM injection
at home.
NIVESTIM is sometimes given by injection into a vein. This is called an intravenous
injection and is generally given by a doctor or nurse.
Equipment required for administration
Make sure that you have all the materials you need for your injection:
A new NIVESTIM pre-filled syringe
An alcohol swab
A puncture-resistant sharps container for disposing of used syringes safely.
Where to inject
The best injection sites are:
your abdomen, except for the area around the navel (belly button) or
the front or side of your thighs.
The sites are shown in the picture below.

You should change the site of injection each time you inject, to avoid soreness at
one site.
Things to do before you inject
Follow these instructions exactly to help avoid contamination and possible infection.
If you are unsure, check with your doctor, nurse or pharmacist.
1. Find a clean, flat working surface, such as a table, where you can inject undisturbed.
2. Remove the carton containing the NIVESTIM pre-filled syringes from the refrigerator.
3. Remove the blister tray containing the pre-filled syringe from the carton. When the
carton contains blister trays with more than one pre-filled syringe, tear off the
blister tray containing one pre-filled syringe along the perforated part, and return
the rest of the blister trays containing pre-filled syringes to the carton and return
the carton to the refrigerator.
4. Open the blister tray containing the pre-filled syringe by peeling away the lid from
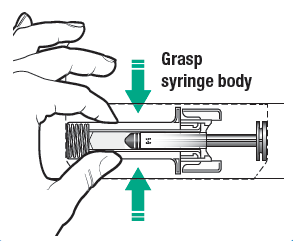
the blister tray. Remove the pre-filled syringe from the blister tray by grasping
from the syringe body.
a. Do not grasp the grey needle cover or the plunger rod.
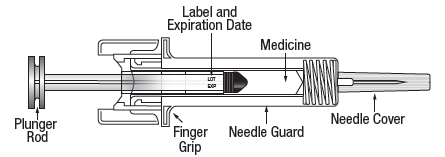
5. Check the syringe to make sure that the needle guard is covering the barrel of the
pre-filled syringe. Do not push the needle guard over the needle cover before the injection. This may activate
or lock the needle guard. If the needle guard is covering the needle that means it
has been activated.
6. Check that the solution is clear, colourless and practically free from visible particles.
Do not inspect the product through the plastic of the safety device.
7. Check the date on the syringe label to make sure that the medicine has not passed
the expiry date.
8. For a more comfortable injection allow the pre-filled syringe to reach room temperature
(approximately 25°C). This will take 15-30 minutes.
a. Do not warm NIVESTIM in any other way (e.g. do not warm it in the microwave or in hot water).
b. Do not shake the syringe.
c. Do not remove the needle cover until you are ready to inject.
9. Make sure you have your puncture-resistant sharps container nearby.
10. Wash and dry your hands thoroughly.
Do not use the NIVESTIM syringe if:
The carton is open or damaged.
The needle guard is missing, detached or has been activated.
The medicine is cloudy or discoloured or the liquid has particles floating in it.
Any part of the pre-filled syringe appears cracked or broken or any of the liquid
has leaked out of the syringe.
The pre-filled syringe has been dropped. The pre-filled syringe may be broken even
if you cannot see the break.
The needle cover is missing or not securely attached.
The expiration date printed on the label has passed.
In all cases above, discard the pre-filled syringe and use a new pre-filled syringe.
How to prepare your injection – NIVESTIM Ready to Use Syringe
1. Hold the pre-filled syringe by the body of the needle guard with the needle cover
pointing up – this helps reduce the amount of medicine that may leak out of the needle.
a. Do not hold by the plunger head, or plunger or needle cover.
b. Do not pull back on the plunger at any time.
c. Do not remove the needle cover from the pre-filled syringe until you are ready to inject
your medicine.
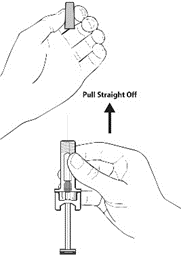
2. Carefully remove the needle cover by holding the barrel and pulling the cover straight
off and away from your body carefully without twisting it. Throw away the cover. Do not recap the needle. Do not push the plunger or touch the exposed needle or shake the syringe.
3. Check the dose (in mL) that your doctor has prescribed and locate the correct volume
mark on the syringe barrel. Carefully push the plunger until the grey upper edge of
the plunger reaches the correct volume mark. This will push the air and any excess
liquid out of the syringe.
4. Double-check that you have the correct dose.
How to inject
1. Clean the site where the injection is to be made with an alcohol swab, moving the
alcohol swab in an expanding circle and allow the site to dry.

2. Pinch a large area of skin between your thumb and forefinger, to create a firm injection
site.
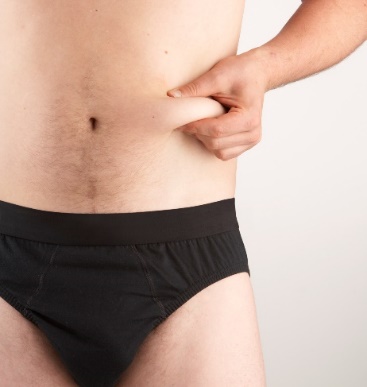
3. With your other hand, pick up the pre-filled syringe and hold it as you would a pencil.
4. Use a quick "dart-like" motion to insert the needle directly into the skin (at an
angle of 45° or as advised by your doctor, nurse or pharmacist).
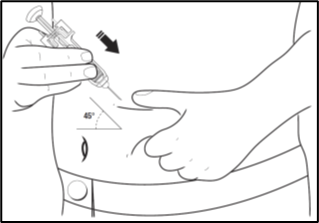
5. After the needle is in, pull back the plunger very slightly. If blood comes into the
syringe, the needle has entered a blood vessel. Remove the needle.
6. Select another site, clean the new site with an alcohol swab and reinsert the needle.
Again, pull back the plunger very slightly to check for blood. If blood does not appear
in the syringe, you are ready to inject.
7. Gently push down the plunger until all the contents of the pre-filled syringe have
been emptied.
8. Withdraw the needle and using the alcohol swab apply pressure for several minutes
to the injection site.
9.
Do not put the needle cover back on the used syringe. You cannot reuse the syringe.
10.
Ensure needle guard covers the needle according to instructions for Active Needle
Guard or Passive Needle Guard (below).
11. Discard the used syringe into an approved, puncture-resistant, sharps container.
Use of Active Ultrasafe Needle Guard for NIVESTIM 120 µg/0.2mL solution for injection
The pre-filled syringe has an UltraSafe Active Needle Guard attached in order to protect
from needle stick injury. When handling the pre-filled syringe, keep hands behind
the needle.
1. Perform the injection using the technique described above.
2. When you have completed the injection, slide the needle guard forward until the needle
is completely covered (device ‘clicks’ into place).
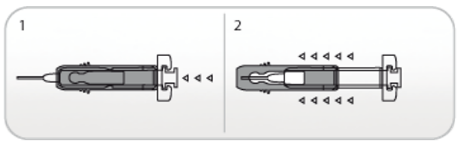
Use of Ultrasafe Passive Needle Guard for NIVESTIM 300 µg/0.5mL solution for injection
and NIVESTIM 480 µg/0.5mL solution for injection
The pre-filled syringe has an UltraSafe Passive Needle Guard attached in order to
protect from needle stick injury. When handling the pre-filled syringe, keep hands
behind the needle.
1. Perform the injection using the technique described above.
2. Depress the plunger while grasping the finger flange until the entire dose has been
given. The passive needle guard will NOT activate unless the ENTIRE dose has been
given.
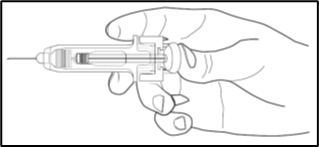
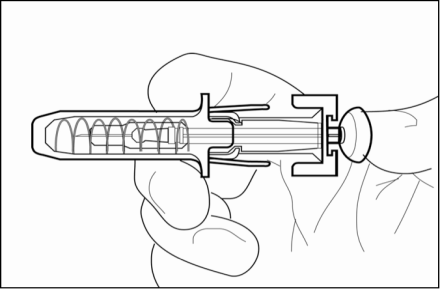
3. Remove needle from your skin, then let go of the plunger and allow syringe to move
up until the entire needle is guarded and locks into place.
Do not change the dose or the way you inject NIVESTIM without consulting your doctor.
Always follow your doctor’s instructions.
How long to use NIVESTIM for
Patients receiving chemotherapy or who have received a bone marrow or stem cell transplant
are only required to use NIVESTIM for short periods of time until the number of infection-fighting
neutrophils are restored (usually 1 to 3 weeks).
Stem cell donors should receive NIVESTIM treatment for 4 to 5 days.
Patients with severe chronic neutropenia are required to use NIVESTIM regularly and
for a long period of time, to keep the number of infection-fighting neutrophils at
a normal level.
Patients with HIV infection need to use NIVESTIM daily until their neutrophil numbers
are normal. Usually, the dose is then reduced to three injections per week to maintain
the neutrophil numbers. Your doctor will tell you how many injections you need each
week and on which days they should be given.
If you forget to use NIVESTIM
If you miss your scheduled dose, inject it as soon as you can – provided that it is
still on the same day.
If you miss a whole day before you remember to inject yourself, do not take a ‘catch-up’
dose or increase your next dose. Advise your doctor, nurse or pharmacist as soon as
possible about the missed dose.
If you use too much NIVESTIM
If you think that you have injected more than the dose recommended by your doctor,
you may need urgent medical attention.
Too much NIVESTIM may lead to neutrophil levels that are too high.
You should immediately:
phone the Poisons Information Centre
(by calling
13 11 26), or
contact your doctor, or
go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using NIVESTIM?
Things you should do
Remind any doctor, dentist, pharmacist or nurse you visit that you are using NIVESTIM,
especially if you are about to be started on any new medicine.
Keep all of your doctor’s appointments so that your health can be monitored. Treatment
with NIVESTIM leads to changes in the numbers of certain blood cells. Your doctor
may order blood tests to check the levels of infection-fighting neutrophils and other
blood cells. Blood tests may also be undertaken after you have completed your NIVESTIM
treatment until your blood cells have returned to normal levels.
Call your doctor straight away if you:
become pregnant during treatment with NIVESTIM.
Go straight to the hospital if you:
Notice any signs or symptoms of an infection.
There are many ways an infection may show itself. You should watch for:
fever (a temperature of 38.2°C or greater, or as your doctor suggests)
chills
rash
sore throat
diarrhoea
earache
difficult or painful breathing, coughing or wheezing.
Things you should not do
Do not stop using this medicine or lower the dosage without checking with your doctor.
Do not use NIVESTIM to treat any other complaint unless your doctor tells you to.
Do not give NIVESTIM to anyone else, even if they have the same condition as you.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how NIVESTIM
affects you.
You may experience dizziness after receiving NIVESTIM and it might affect your ability
to drive or use machines.
Looking after your medicine
Keep NIVESTIM in a refrigerator at a temperature of 2°C to 8°C. Keep it in its carton
protected from light.
Brief exposure to freezing temperatures (up to 24 hours) will not harm NIVESTIM, nor
will exposure to room temperature for up to fifteen (15) days in a single period.
Do not use NIVESTIM if it has been left out of the refrigerator for more than fifteen
(15) days, or if it has been in the freezer for more than 24 hours, or if it has been
frozen more than once.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do
not store it:
in the bathroom or near a sink, or
in the car or on window sills.
Keep it where young children cannot reach it.
When to discard your medicine
Once you have injected NIVESTIM, do not put the needle cover back on the used syringe.
Put the used syringe into an approved, puncture-resistant, sharps container. Dispose
of the full puncture-resistant sharps container as instructed by your doctor, nurse
or pharmacist.
Never put used syringes into your normal household rubbish bin.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy
for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of
them are minor and temporary. However, some side effects may need medical attention.
Tell your doctor as soon as possible if you have any problems while using NIVESTIM,
even if you do not think the problems are connected with the medicine or are not listed
in this leaflet.
See the information below and, if you need to, ask your doctor or pharmacist if you
have any further questions about side effects.
Serious side effects
|
Serious side effects
|
What to do
|
|
General:
chest pain
fever
general feeling of tiredness
easy bruising or bleeding
left shoulder tip pain
frequent infections
Dizziness or feeling light-headed
Faintness
Lungs and upper airways:
coughing up blood or mucus, bleeding from the lung
breathing problems such as shortness of breath, rapid breathing
Gut, Digestion and Urine-related:
pain in the upper left side of the stomach (abdomen)
swelling of your stomach-area (abdomen) and feeling of fullness
less frequent urination
blood in the urine
Skin:
fever and painful skin lesions, most commonly on your arms, legs and sometimes on
your face and neck
swelling or puffiness
Heart-related:
fever, chest or abdominal pain, malaise and back pain. These could be symptoms of
inflammation of your aorta (the large vessel that transports blood from your heart
to your body). Tell your doctor if you experience these symptoms.
Symptoms of severe allergic reaction:
pinkish, itchy swellings on the skin, also called hives or nettle rash
swelling of the face, lips, mouth or throat which may cause difficulty in swallowing
or breathing
shortness of breath, wheezing
light-headedness, dizziness or fainting
rapid pulse, sweating
|
Call your doctor straight away, or go straight to the Emergency Department at your
nearest hospital if you notice any of these serious side effects.
|
Tell your doctor or pharmacist if you notice anything else that may be making you
feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can
report side effects to the Therapeutic Goods Administration online at
www.tga.gov.au/reporting-problems . By reporting side effects, you can help provide more information on the safety of
this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop
taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What NIVESTIM contains
|
Active ingredient
(main ingredient)
|
filgrastim (rbe)
|
|
Other ingredients
(inactive ingredients)
|
glacial acetic acid
polysorbate 80
sodium hydroxide
sorbitol
water for injections
|
Do not take this medicine if you are allergic to any of these ingredients.
Each pre-filled syringe is affixed with a needle closed by a needle cover that contains
epoxyprene, a derivative of natural rubber latex which may come into contact with
the needle.
NIVESTIM does not contain lactose, gluten, tartrazine or any other azo dyes.
What NIVESTIM looks like
NIVESTIM is a clear, colourless solution and is supplied as ready-to-use syringes.
The single use, preservative-free syringes are packed in cartons of 1, 5, or 10 and
are available in the following strengths:
120 micrograms of filgrastim in a volume of 0.2 mL; (AUST R 160106).
300 micrograms of filgrastim in a volume of 0.5 mL; (AUST R 160108).
480 micrograms of filgrastim in a volume of 0.5 mL (AUST R 160107).
Who distributes NIVESTIM
Pfizer Australia Pty Ltd
Sydney NSW
This leaflet was prepared in December 2025.
® = Registered Trademark