2. What should I know before I use ORFADIN?
Do not use if you have ever had an allergic reaction to ORFADIN or any of the ingredients
listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines,
or are pregnant or plan to become pregnant or are breastfeeding.
3. What if I am taking other medicines?
Some medicines may interfere with ORFADIN and affect how it works.
4. How do I use ORFADIN?
The oral suspension is taken with an oral syringe directly in the mouth without dilution.
ORFADIN must not be injected. Do not attach a needle to the syringe.
Your doctor will prescribe the correct dose for you. Your doctor will adjust your
ORFADIN dose based on your response to ORFADIN and your test results.
5. What should I know while using ORFADIN?
|
Things you should do
|
Remind any doctor, dentist or pharmacist you visit that you are using ORFADIN.
It is very important that you stay on the special diet, with low tyrosine and phenylalanine
content that your doctor recommended.
Tell your doctor if you are pregnant or breastfeeding, or intend to become pregnant
or breastfeed.
Tell your doctor immediately if you experience red eyes, clouding or inflammation
of the eyes, inflammation of the eyelid, sensitivity to light, or eye pain.
|
|
Things you should not do
|
Do not stop using this medicine or lower the dosage without checking with your doctor.
Do not breastfeed while taking ORFADIN.
|
|
Driving or using machines
|
Be careful driving or operating machinery or doing other jobs until you know how ORFADIN
affects you. ORFADIN is not known to cause dizziness or light headedness, but ORFADIN
can affect your vision if you develop certain side effects.
|
|
Looking after your medicine
|
Keep your oral suspension in the original bottle until it is time to take it.
Keep your unopened oral suspension bottle in its box, in a refrigerator between 2°C
- 8°C. Do not freeze. Store the bottle upright.
|
6. Are there any side effects?
If you do experience any side effects, most of them are minor and temporary. Tell
your doctor immediately if you experience red eyes, clouding or inflammation of the
eyes, inflammation of the eyelid, sensitivity to light, or eye pain. Call your doctor
straight away or go to the Emergency Department at your nearest hospital if you experience
shortness of breath, wheezing or difficulty breathing, or swelling of the face, lips,
tongue or other parts of the body.
For more information, including what to do if you have any side effects, see Section
6. Are there any side effects? in the full CMI.
Active ingredient(s):
nitisinone
Full Consumer Medicine Information (CMI)
This leaflet provides important information about using ORFADIN. You should also speak to your doctor or pharmacist if you would like further information
or if you have any concerns or questions about using ORFADIN.
Where to find information in this leaflet:
1. Why am I using ORFADIN?
ORFADIN contains the active ingredient nitisinone.
Nitisinone belongs to a group of medicines called 'other alimentary and metabolism
products'.
ORFADIN is used for the treatment of a disease called hereditary tyrosinaemia type
1 (HT-1).
In this disease the body is unable to completely break down the amino acid tyrosine.
Harmful substances can form and accumulate in the body.
ORFADIN blocks the breakdown of tyrosine and by doing so the harmful substances are
not formed. However, tyrosine will remain in the body and therefore a special diet
that is low in tyrosine and phenylalanine content must be followed when taking ORFADIN.
Ask your doctor if you have any questions about why this medicine has been prescribed
for you.
Your doctor may have prescribed it for another reason.
2. What should I know before I use ORFADIN?
Diet
Tell your doctor about your diet regimen.
Your doctor will advise you on how to maintain a diet restricted in tyrosine and phenylalanine
content.
Do not use ORFADIN if:
you are allergic to nitisinone, or any of the ingredients listed at the end of this
leaflet.
Always check the ingredients to make sure you can use this medicine.
Do not take this medicine after the expiry date printed on the pack or if the packaging
is torn or shows signs of tampering.
If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Check with your doctor if you:
have any other medical conditions
take any medicines for any other condition
During treatment, you may be at risk of developing certain side effects. It is important
you understand these risks and how to monitor for them. See additional information
under Section
6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
Your doctor can discuss with you the risks and benefits involved.
Do not breastfeed if you are taking this medicine.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any
medicines, vitamins or supplements that you buy without a prescription from your pharmacy,
supermarket or health food shop.
Some medicines may interfere with ORFADIN and affect how it works. These include:
warfarin, a medicine used to thin your blood
phenytoin, a medicine used to treat epilepsy
furosemide (frusemide), a diuretic
These medicines may be affected by ORFADIN or may affect how well it works. You may
need different amounts of your medicines, or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or
avoid while taking ORFADIN.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins
or supplements you are taking and if these affect ORFADIN.
4. How do I use ORFADIN?
Follow all directions given to you by your doctor or pharmacist carefully.
They may differ from the information contained in this leaflet.
The oral suspension is taken with an oral syringe directly in the mouth without dilution.
ORFADIN must not be injected.
Do not attach a needle to the syringe.
If you do not understand the instructions, ask your doctor or pharmacist for help.
Your doctor or pharmacist can advise you how to use the oral syringe correctly.
How much to take
The recommended starting dose is 1 mg/kg body weight/day divided in 2 doses administered
orally. Your doctor will prescribe the correct dose for you.
Your doctor may change your total dose from twice a day to once a day if you weigh
more than 20 kg and you have been taking ORFADIN twice daily for at least 4 weeks.
Your doctor will adjust your ORFADIN dose based on your response to ORFADIN and your
test results.
When to take ORFADIN
If you are taking ORFADIN twice daily, take your first dose in the morning and your
second dose in the evening.
Your doctor will advise you on the correct time to take ORFADIN when moving you to
once daily dose.
Take ORFADIN at about the same time each day.
Taking it at the same time each day will have the best effect. It will also help you
remember when to take it.
How to take ORFADIN
Take ORFADIN with food.
How to prepare the dose to be administered
The dose that your doctor prescribes you should be given in mL of suspension and not
in mg. This is because the oral syringe which is used to withdraw the correct dose
from the bottle is marked in mL.
If your prescription is in mg, contact your pharmacist or doctor for advice.
The pack contains a bottle of medicine with a cap, a bottle adaptor and three oral
syringes (1.5 mL, 3 mL and 6 mL). Always use one of the oral syringes provided to
take the medicine.
The 1.5 mL oral syringe (the smallest oral syringe) is marked from 0.1 mL to 1.5 mL
with minor 0.05 mL graduations. It is used for measuring doses of up to 1.5 mL.
The 3 mL oral syringe (the middle sized oral syringe), is marked from 1 mL to 3 mL
with minor 0.1 mL graduations. It is used for measuring doses of more than 1.5 mL
and up to 3 mL.
The 6 mL oral syringe (the largest oral syringe), is marked from 1 mL to 6 mL with
minor 0.25 mL graduations. It is used for measuring doses of more than 3 mL.
It is important that you use the correct oral syringe when taking the medicine. Your
doctor, pharmacist or nurse will advise which oral syringe to use depending on the
dose that has been prescribed.
Follow the instructions given below for dose preparation and administration carefully,
in order to ensure that the correct dose is administered.
How to prepare a new bottle of ORFADIN oral suspension before first time use
Before taking the first dose, shake the bottle vigorously since during long-term storage
the particles will form a solid cake at the bottom of the bottle.
1. Remove the bottle from the refrigerator. Write the date when the bottle should be
discarded in the space provided on the bottle label, which is 2 months after it is
first opened
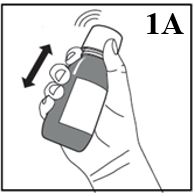
Figure 1A
2. Before measuring the first dose, shake the unopened bottle vigorously for AT LEAST
20 SECONDS until the solid cake at the bottom of the bottle is completely dispersed
(Figure 1A).
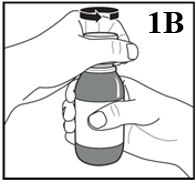
Figure 1B
3. Remove the child resistant screw cap by pushing it down firmly and turning it anti-clockwise
(Figure 1B).
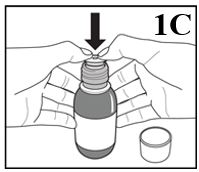
Figure 1C
4. Place the open bottle upright on a table. Push the plastic adaptor firmly into the
neck of the bottle as far as you can (Figure 1C). Leave the plastic adaptor in place
for the duration of the use of this bottle of medicine. Once the plastic adaptor is
firmly in place, if you do not intend to administer the first dose now, close the
bottle by screwing the child resistant cap back onto the bottle. If you intend to
administer the first dose now, do not close the bottle but instead follow the instructions
below from Step 3 (Figure 2C) onwards.
Follow the instructions below for all subsequent dosing.
How to prepare and administer ORFADIN oral suspension for subsequent dosing
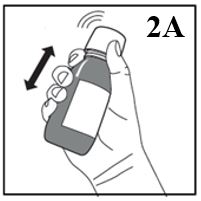
Figure 2A
1. Shake the bottle vigorously for AT LEAST 5 SECONDS (Figure 2A).
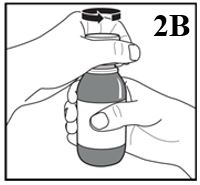
Figure 2B
2. Immediately thereafter, open the bottle by removing the child resistant screw cap
(Figure 2B).
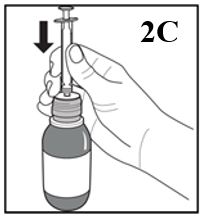
Figure 2C
3. Push the syringe plunger down completely
4. Keep the bottle in an upright position and insert the oral syringe firmly into the
hole of the adaptor on the bottle (Figure 2C).
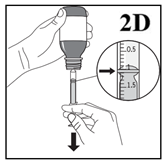
Figure 2D
5. Carefully turn the bottle upside down with the oral syringe firmly in place (Figure
2D).
6. In order to withdraw the prescribed dose (mL), pull the plunger back SLOWLY until
the top edge of the plunger is exactly level with the line marking the required dose
(Figure 2D). If any air bubbles are observed inside the filled oral syringe, push
the plunger into the syringe barrel until the air bubbles are expelled. Then pull
the plunger back again until the top edge is exactly level with the line marking the
dose.
7. Turn the bottle to an upright position again. Disconnect the oral syringe by gently
twisting it out of the bottle adaptor.
8. The dose should be administered in the mouth immediately (without dilution) in order
to avoid caking in the oral syringe. The oral syringe must be emptied SLOWLY to allow
swallowing; rapid squirting of the medicine may cause choking.
9. Immediately screw the child resistant cap onto the bottle. The bottle adaptor should
not be removed.
10. The opened bottle of medicine may be stored at a temperature below 25°C or in the
refrigerator (2-8°C) and must be discarded 2 months after opening. The date it should
be discarded should be noted on the bottle label.
Cleaning
Clean the oral syringe IMMEDIATELY after use with cold tap water only, and if necessary,
move the plunger in and out. Shake off excess water and leave the oral syringe to
dry until it is required for the next dosing occasion. Do not disassemble the oral
syringe.
How long to take ORFADIN
Continue taking ORFADIN for as long as your doctor tells you.
This medicine helps to control your condition, but does not cure it. It is important
to keep taking your medicine even if you feel well.
If you forget to take ORFADIN
Do not take a double dose to make up for the dose that you missed.
This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some
hints.
If you take too much ORFADIN
If you think that you have taken too much ORFADIN, you may need urgent medical attention.
You should immediately:
phone the Poisons Information Centre
(by calling
13 11 26), or
contact your doctor, or
go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using ORFADIN?
Things you should do
It is very important that you stay on the special diet, with low tyrosine and phenylalanine
content that your doctor recommended. If you do not understand the instructions on
your diet, ask your doctor for help.
If you are about to be started on any new medicine, remind your doctor and pharmacist
that you are taking ORFADIN.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking
ORFADIN. Remind them if any new medicines are about to be started.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking
ORFADIN.
It may affect other medicines used during surgery.
If you become pregnant while taking this medicine, tell your doctor immediately.
If you are about to have any blood tests, tell your doctor that you are taking this
medicine.
It may interfere with the results of some tests.
Keep all of your doctor's appointments so that your progress can be checked.
Your doctor will do regular blood and urine checks to ensure that you are on the right
dose of ORFADIN and to make sure that there are no possible side effects causing blood
disorders.
Your doctor will also check your liver regularly as the disease can affect the liver.
Your doctor will also check on your general development.
Call your doctor straight away if you:
If you experience red eyes, or any of the following eye problems, tell your doctor
immediately.
Your doctor will immediately check your eyes.
Eye problems could be a sign of inadequate dietary control.
Different eye symptoms as listed below:
scarring or clouding of the cornea
inflammation in the cornea
inflammation in the eye
inflammation in the eyelid
sensitivity to light
eye pain
Remind any doctor, dentist or pharmacist you visit that you are using ORFADIN.
Things you should not do
ORFADIN must not be injected.
Do not attach a needle to the syringe.
Do not take ORFADIN to treat any other complaints unless your doctor tells you to.
Do not give ORFADIN to anyone else, even if they have the same condition as you.
Do not stop taking ORFADIN or lower the dosage without checking with your doctor.
If you stop taking it suddenly, your condition may worsen or you may have unwanted
side effects.
Do not breastfeed while taking ORFADIN.
Driving or using machines
Be careful driving or operating machinery or doing other jobs until you know how ORFADIN
affects you.
ORFADIN is not known to cause dizziness or light headedness, but ORFADIN can affect
your vision if you develop certain side effects.
Looking after your medicine
Keep your oral suspension in the original bottle until it is time to take it.
If you take the oral suspension out of the bottle, it may not keep well.
Keep your unopened oral suspension bottle in its box, in a refrigerator between 2°C
- 8°C. Do not freeze. Store the bottle upright.
After opening, keep the bottle in its box in a cool place where the temperature stays
below 25°C, for a single period of 2 months.
Do not store ORFADIN or any other medicine in the bathroom or near a sink. Do not
leave it on a window sill or in the car.
Heat and dampness can destroy some medicines.
Keep it where young children cannot reach it.
A locked cupboard at least one-and-a-half metres above the ground is a good place
to store medicines.
When to discard your medicine
ORFADIN Oral suspension must be discarded 2 months after opening.
To help you remember, write the discard date in the space provided on the ORFADIN
bottle when you first open the bottle.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy
for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of
them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you
have any further questions about side effects.
Less serious side effects
Serious side effects
Tell your doctor or pharmacist if you notice anything else that may be making you
feel unwell.
Other side effects not listed here may occur in some people.
There may be other side effects affecting your liver and certain blood cell counts
that your doctor will carefully monitor by doing regular blood and urine checks.
Some of these side effects can only be found when your doctor does tests from time
to time to check your progress.
Reporting side effects
After you have received medical advice for any side effects you experience, you can
report side effects to the Therapeutic Goods Administration online at
www.tga.gov.au/reporting-problems . By reporting side effects, you can help provide more information on the safety of
this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop
taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
ORFADIN is not addictive.
What ORFADIN contains
|
Active ingredient
(main ingredient)
|
Each mL contains 4 mg nitisinone
|
|
Other ingredients
(inactive ingredients)
|
Glycerol
Citric acid monohydrate
Hypromellose
Sodium citrate
Sodium benzoate
Flavour: Strawberry 501440 T (Proprietary Ingredient Number 11565)
Polysorbate 80
Purified water
|
|
Potential allergens
|
None
|
Do not take this medicine if you are allergic to any of these ingredients.
This medicine does not contain lactose, sucrose, gluten, tartrazine or any other azo
dyes.
What ORFADIN looks like
ORFADIN oral suspension is a white, slightly thicker opaque suspension. Before shaking
the bottle, it may look like a solid cake in the bottom and a slightly opalescent
liquid.
ORFADIN oral suspension is provided in a 100 mL brown glass bottle with a white, child
resistant screw cap.
Each bottle contains 90 mL suspension.
Each pack contains one bottle, one bottle adaptor and three oral syringes.
Who distributes ORFADIN
ORFADIN is supplied by:
A. Menarini Australia Pty. Ltd.
Sydney, Australia
Medical Information: 1800 644 542
Australian Registration Number:
ORFADIN Oral Suspension 4 mg/mL: AUST R 297736
ORFADIN® is a registered trademark of Swedish Orphan Biovitrum International AB, used
under licence by A. Menarini Australia Pty. Ltd.
This leaflet was prepared in September 2025.
[vA04-0]