2. What should I know before I use Somatuline Autogel?
Do not use if you have ever had an allergic reaction to lanreotide or any of the ingredients
listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines,
or are pregnant or plan to become pregnant or are breastfeeding.
3. What if I am taking other medicines?
Some medicines may interfere with Somatuline Autogel and affect how it works.
4. How do I use Somatuline Autogel?
The recommended starting dose is 60 mg to 120 mg every 28 days. Your doctor will decide
on the right dose for you.
You must follow all the instructions at the end of this leaflet when using Somatuline
Autogel.
5. What should I know while using Somatuline Autogel?
|
Things you should do
|
Remind any doctor, dentist or pharmacist you visit that you are using Somatuline Autogel.
Tell your doctor if you are breastfeeding.
Tell your doctor if you have diabetes or any heart problems.
|
|
Things you should not do
|
Do not stop using this medicine suddenly.
|
|
Driving or using machines
|
Be careful before you drive or use any machines or tools.
This medicine may cause dizziness in some people.
|
|
Looking after your medicine
|
Store Somatuline Autogel at 2°C - 8°C in a refrigerator in its original package. Do
not freeze.
|
6. Are there any side effects?
Like all medicines, Somatuline Autogel can cause side effects, although not everybody
gets them. For more information, including what to do if you have any side effects,
see Section
6. Are there any side effects? in the full CMI.
Some side effects may need medical attention, tell your doctor straight away if you
notice any of the following:
severe and sudden abdominal pain, yellowing of the skin and eyes, high fever, feeling
nauseous, vomiting, dark urine or clay-coloured stools. These may be signs of gallbladder
stones (very common), or gallstone complications (uncommon).
feeling confused, shaky, more hungry or sweating more than usual, more thirsty or
tired than usual, dry mouth. These may be signs of low or high blood sugar levels
or development of diabetes (common).
Active ingredient(s):
Lanreotide
Full Consumer Medicine Information (CMI)
This leaflet provides important information about using Somatuline Autogel. You should also speak to your doctor or pharmacist if you would like further information
or if you have any concerns or questions about using Somatuline Autogel.
Where to find information in this leaflet:
1. Why am I using Somatuline Autogel?
Somatuline Autogel contains the active ingredient lanreotide. Lanreotide is an octapeptide, an analogue of a naturally occurring hormone, somatostatin.
Lanreotide lowers the levels of hormones in the body such as GH (growth hormone) and
IGF-1 (insulin-like growth factor-1).
Somatuline Autogel is used for:
the treatment of acromegaly (a condition where your body produces too much growth
hormone),
the relief of symptoms such as flushing and diarrhoea that sometimes occur in patients
with neuroendocrine tumours (NETs),
the treatment and control of the growth of some advanced tumours of the intestine
and pancreas called gastroenteropancreatic neuroendocrine tumours (GEP-NETs). It is
used when these tumours cannot be removed by surgery.
2. What should I know before I use Somatuline Autogel?
Warnings
Do not use Somatuline Autogel if:
you are breastfeeding.
you are allergic to lanreotide, or any of the ingredients listed at the end of this
leaflet.
Always check the ingredients to make sure you can use this medicine.
Some of the symptoms of an allergic reaction may include:
shortness of breath
wheezing or difficulty breathing
swelling of the face, lips, tongue, or other parts of the body
rash, itching or hives on the skin
Check with your doctor if you:
are a diabetic
have ever experienced liver or kidney problems
have ever experienced gallstones
have any thyroid problems
have any heart problems
take any medicines for any other condition
During treatment, you may be at risk of developing certain side effects. It is important
you understand these risks and how to monitor for them. See additional information
under Section
6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
Do not breastfeed if you are taking Somatuline Autogel.
Use in children
Somatuline Autogel is not recommended for use in children.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any
medicines, vitamins or supplements that you buy without a prescription from your pharmacy,
supermarket or health food shop.
Some medicines and Somatuline Autogel may interfere with each other, these include
(but are not limited to):
Medicines used to help prevent transplant rejection or treat problems with the immune
system, such as ciclosporin.
Medicines used to treat Parkinson’s disease, such as bromocriptine.
Medicines known as beta-blockers, that are used to treat high blood pressure, heart
conditions, glaucoma, and migraine.
Medicines that are broken down in the body by liver enzymes, such as quinidine or
terfenadine.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins
or supplements you are taking and if these affect Somatuline Autogel.
4. How do I use Somatuline Autogel?
How much to use
Acromegaly or symptoms associated with NETs:
The recommended starting dose is 60 mg to 120 mg injected every 28 days.
GEP-NETs:
The recommended dose is 120 mg every 28 days.
Depending on your response to the medicine, your doctor may vary the dose or the injection
frequency.
When to use Somatuline Autogel
Somatuline Autogel should be used once every 28 days.
Your doctor will decide on the length of your treatment with Somatuline Autogel.
Use Somatuline Autogel until your doctor tells you to stop.
How to use Somatuline Autogel
You must follow the instructions at the end of this leaflet.
It is intended for deep injection under the skin.
It is for single use only.
Your doctor may decide that you or your carer can administer the injection. Your doctor
or nurse will give you detailed instructions on how to do this. It is important not
to try to inject yourself until you have been trained by a healthcare professional
and you are sure of how to do it.
Follow all directions given to you by your doctor, nurse or pharmacist carefully.
These may differ from the information contained in this leaflet.
This is a long-term treatment. Keep using this medicine for as long as your doctor
tells you.
Your doctor will regularly monitor your condition to check that the treatment is having
the desired effect.
If the injection is being given by your doctor, nurse or carer, the injection will
usually be given in the upper, outer external quadrant of the buttock.
If you are giving the injection to yourself, the injection should be made in the upper,
outer thigh.
The injection site should be alternated between right and left sides with each injection.
Follow the instructions at the end of this leaflet.
If you forget to use Somatuline Autogel
If you miss your dose at the usual time, contact your doctor who will advise when
your next injection should be given.
Do not inject a double or extra dose to make up for the dose you missed.
If you use too much Somatuline Autogel
This medicine is given to you under the supervision of your doctor, so it is very
unlikely that you will receive too much. The medicine comes in a syringe pre-filled
with the dose your doctor has prescribed.
However, if you feel you have been given too much, you may need urgent medical attention.
You should immediately:
phone the Poisons Information Centre
(by calling
13 11 26), or
contact your doctor, or
go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using Somatuline Autogel?
Things you should do
Call your doctor straight away if you:
feel more thirsty or tired than usual and have a dry mouth. These may be signs that
you have high blood sugar levels or are developing diabetes.
feel hungry, shaky, sweating more than usual or feel confused. These may be signs
of low blood sugar levels.
Remind any doctor, dentist or pharmacist you visit that you are using Somatuline Autogel.
Talk to your doctor or pharmacist if you:
have fatty stools, loose stools, abdominal bloating, or weight loss. These may be
signs of pancreatic exocrine insufficiency (PEI), a condition where your pancreas
doesn't make enough digestive enzymes used to break down food for your body to absorb.
Things you should not do
Do not stop using this medicine suddenly.
Diabetes
If you are diabetic, your doctor may check your blood sugar levels and possibly alter
your anti-diabetic treatment while you are receiving Somatuline Autogel.
Heart problems
If you have heart problems, your doctor may check your heart rate and possibly alter
your treatment while you are taking Somatuline Autogel.
Gallbladder problems
Due to the possibility of gallbladder problems with this type of medicine, your doctor
may want to conduct a gallbladder scan when you start receiving Somatuline Autogel
and again at regular intervals thereafter.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how Somatuline
Autogel affects you.
This medicine may cause dizziness in some people.
Looking after your medicine
Store Somatuline Autogel at 2°C - 8°C in a refrigerator in its original package.
Do not freeze.
The syringe in its sealed pouch can be temporarily stored outside of the refrigerator
on up to three occasions for a maximum of 72 hours in total (make sure the temperature
stays below 40°C). Return the syringe to the refrigerator as soon as possible for
continued storage and use.
Follow the instructions in the carton on how to take care of your medicine properly.
Do not use it if the laminated pouch is damaged or opened.
Keep it where young children cannot reach it.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy
for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of
them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you
have any further questions about side effects.
Less serious side effects
Tell your doctor or pharmacist if you notice anything else that may be making you
feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can
report side effects to the Therapeutic Goods Administration online at
www.tga.gov.au/reporting-problems . By reporting side effects, you can help provide more information on the safety of
this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop
taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What Somatuline Autogel contains
|
Active ingredient
(main ingredient)
|
Lanreotide acetate
|
|
Other ingredients
(inactive ingredients)
|
Sterile water
Acetic acid
|
|
Potential allergens
|
n/a
|
Do not take this medicine if you are allergic to any of these ingredients.
What Somatuline Autogel looks like
Somatuline Autogel is a pre-filled syringe is packed in a laminated pouch and a cardboard
box.
Each box contains one 0.5 mL syringe with an automatic safety system and one needle
(1.2 mm x 20 mm). (AUST R 95260, 95261, 95262).
Australian Sponsor of Somatuline Autogel
Ipsen Pty Ltd
Level 5, 627 Chapel Street
South Yarra Victoria 3141
Somatuline® Autogel® is a registered trademark of Ipsen Pharma S.A.S.
This leaflet was prepared in January 2024.
8. Instructions for use
These instructions explain how to inject Somatuline Autogel.
Please read all the instructions carefully before starting the injection.
The injection is a deep subcutaneous injection that requires a specific technique
different to normal subcutaneous injections.
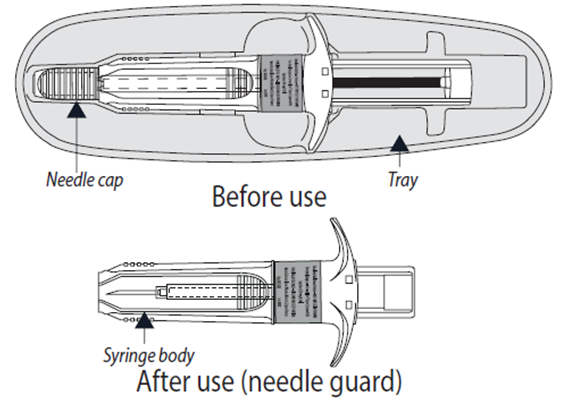
Somatuline Autogel is supplied in a ready to use pre-filled syringe fitted with an
automatic safety system. The needle will retract automatically following the full
administration of the product, to prevent needle stick injury.
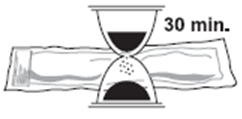
1. Ensure that the medication has been refrigerated in its original package.Remove Somatuline Autogel from the refrigerator 30 minutes prior to administration.
Injection of cold medication may be painful. Keep laminated pouch sealed until just
prior to injection.
2. Before opening the pouch, check that it is intact and that the medication has not
expired. The expiration date is printed on the outer carton and the pouch.
Do not use if:
You drop or damage the pre-filled syringe.
The pre-filled syringe or pouch appear damaged in any way.
The product has expired.
If any of the above apply you should contact your doctor or pharmacist.
3. Wash hands with soap and ensure there is a clean area for preparation.
4. Tear open the pouch along the dotted line and take out the pre-filled syringe. The content of the pre-filled syringe is semi-solid with a gel-like appearance, and
a colour varying from white to pale yellow. The solution can also contain very small
bubbles that can clear up during injection. These differences are normal and do not
interfere with the quality of the product.
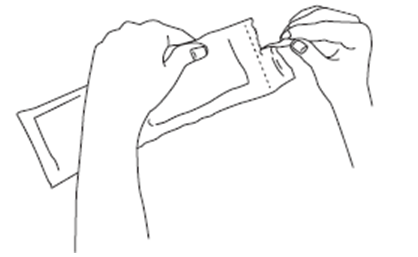
After opening the protective laminated pouch, the product should be administered immediately.
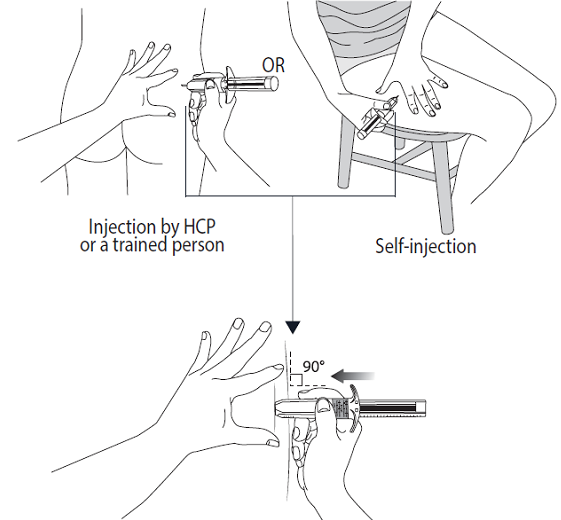
5. Select an injection site:
5a. If a healthcare professional (HCP) or someone else like a trained family member
or friend is doing the injection: use the superior external (upper, outer) quadrant
of the buttock for injection, or
5b. If you are injecting yourself: use the upper outer part of your thigh.
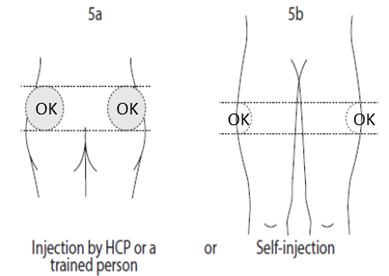
Alternate the injection site between the right and left side each time you receive an injection of Somatuline
Autogel. Avoid areas with moles, scar tissue, reddened skin, or skin that feels bumpy.
6. Clean the injection site without rubbing the skin excessively and let it dry.
7. Before injecting, remove the pre-filled syringe from its tray. Discard the tray.
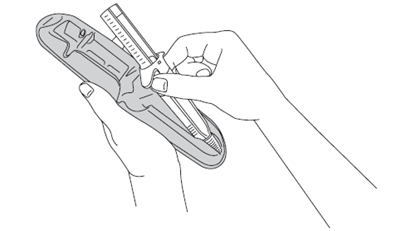
8. Remove the needle cap by pulling it off and discard it.
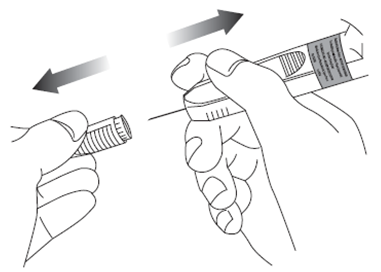
9.
Flatten injection area using the thumb and index finger of the hand not holding the pre-filled
syringe to stretch the skin. Do not pinch the skin. Use a strong, straight dart-like motion to quickly insert the needle perpendicular to the skin (90° angle), all the way into the skin.It is very important that you insert the needle completely. You should not see any needle once it is fully inserted.
Do not aspirate (do not draw back).
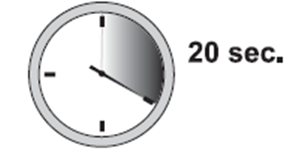
10. Release injection site that has been flattened by your hand. Push plunger with steady very firm pressure. The medication is thicker and harder to push than you might expect. Typically, 20
seconds are needed. Inject the full dose and give a final push to make sure you cannot depress it any further.
Note: maintain pressure on the plunger with your thumb to avoid activation of the
automatic safety system.
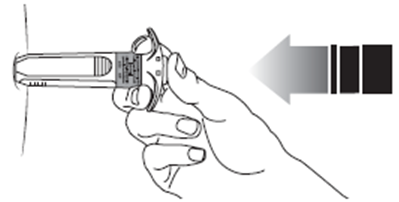
11. Without releasing the pressure on the plunger, withdraw the needle from the injection
site.
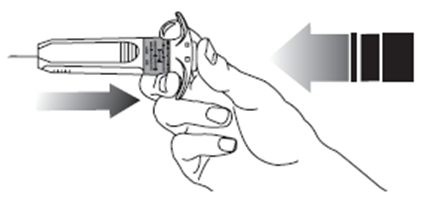
12. Then release the pressure on the plunger. The needle will automatically retract into
the needle guard where it will be locked permanently.
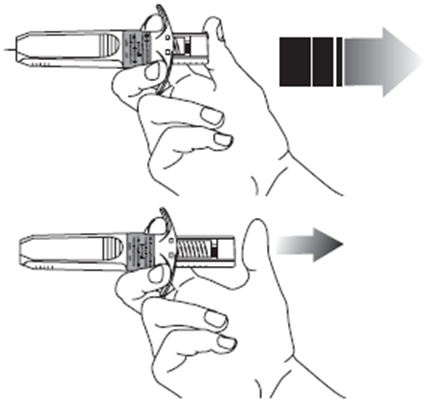
13. Apply gentle pressure to the injection site with a dry cotton ball or sterile gauze
to prevent any bleeding. Do not rub or massage the injection site after administration.
14. Dispose of the used syringe in the syringe disposal container as instructed by your
doctor or healthcare provider. Do not dispose of the device in your general household rubbish.Keep the disposal container and Somatuline Autogel out of reach and sight of children.