2. What should I know before I use SUBUTEX?
Do not use if you have ever had an allergic reaction to SUBUTEX or any of the ingredients
listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines,
or are pregnant or plan to become pregnant or are breastfeeding.
3. What if I am taking other medicines?
Some medicines may interfere with SUBUTEX and affect how it works.
4. How do I use SUBUTEX?
SUBUTEX should be used exactly as prescribed by your doctor or healthcare professional.
The usual starting dose of SUBUTEX is 4 - 8 mg with an additional 4 mg depending on
your needs as determined by your treating doctor. The subsequent dosing will be selected
by your healthcare professional based on clinical assessment of your condition.
5. What should I know while using SUBUTEX?
|
Things you should do
|
Remind any doctor, dentist, nurse or pharmacist you visit that you are using SUBUTEX.
Tell your family or friends that, in the event of emergency, they should inform the
treating healthcare provider or Emergency Room staff that you are being treated with
SUBUTEX.
|
|
Things you should not do
|
Do not inject this medicine.
Do not swallow or consume food or drink until the tablet is completely dissolved.
Do not stop using this medicine suddenly.
|
|
Driving or using machines
|
SUBUTEX may affect your alertness and ability to drive and operate machinery. Be careful
driving or operating machinery until you know how SUBUTEX affects you.
|
|
Drinking alcohol
|
Do not drink alcohol or take medicines that contain alcohol whilst being treated with
SUBUTEX. Alcohol and some medicines may increase the sedative effects of SUBUTEX
|
|
Looking after your medicine
|
Keep the tablets in a dry place where temperature stays below 30°C. Store this medicine
securely, where other people cannot access it. It may harm people who take this medicine
by accident, or intentionally.
|
6. Are there any side effects?
|
WARNINGS:
Hazardous and harmful use
Although SUBUTEX is indicated for the treatment of opioid dependence, it still poses
risks of hazardous and harmful use which can lead to overdose and death. The doctor
will monitor your ongoing risk during treatment with SUBUTEX.
Life threatening respiratory depression
Serious, life-threatening respiratory depression may occur with the use of SUBUTEX.
Talk to your doctor about situations which may increase the risk of respiratory depression.
Concomitant use of medicines affecting the central nervous system, including alcohol
Use of SUBUTEX with anti-anxiety medicines, sedatives, antihistamines, some antidepressants,
antipsychotics, cannabis and alcohol may result in profound sedation, respiratory
depression, coma and death.
|
Active ingredient:
buprenorphine hydrochloride
Full Consumer Medicine Information (CMI)
This leaflet provides important information about using SUBUTEX. You should also speak to your doctor or pharmacist if you would like further information
or if you have any concerns or questions about using SUBUTEX.
Where to find information in this leaflet:
1. Why am I using SUBUTEX?
SUBUTEX contains the active ingredient buprenorphine hydrochloride. Buprenorphine acts as a substitute for opioids like heroin, morphine, oxycodone or
codeine and it helps withdrawal from opioids over a period of time.
SUBUTEX is used as part of a medical, social and psychological treatment program for
patients dependent on opioids like heroin, morphine, oxycodone or codeine.
Ask your doctor or healthcare professional if you have any questions about why SUBUTEX
has been prescribed for you.
2. What should I know before I use SUBUTEX?
Warnings
SUBUTEX is only for adults and children over the age of 16 years.
Opioids can cause
sleep apnoea (stopping breathing from time to time while sleeping) which can lead
to low levels of oxygen in the blood. Tell your doctor if you have a history of
sleep apnoea or if anyone notices you stop breathing from time to time whilst sleeping.
a decreased level of hormones in the blood caused by a problem with the adrenal glands.
The effects of these hormone changes may include nausea, vomiting, loss of appetite,
feeling very tired and weak, feeling dizzy, or low blood pressure.
an increased level of the hormone ‘prolactin’ and decreased level of sex hormones
in the blood.
Tell your doctor if you notice any of these symptoms. You may need blood tests, and
your doctor may tell you to stop using SUBUTEX.
Do not use SUBUTEX if:
you are allergic to buprenorphine or any of the ingredients listed at the end of this
leaflet.
you are taking benzodiazepines unless they have been prescribed by your doctor.
always check the ingredients to make sure you can use this medicine.
you have serious problems with your liver, or if your doctor detects the development
of a serious liver problem during treatment.
you are under the age of 16 years.
you have serious breathing problems.
you are intoxicated due to CNS depressant medicines (e.g. tranquilisers, sedative/hypnotics,
narcotic pain killers, anti-anxiety or antipsychotic medicines), alcohol or have delirium
tremens (the ‘shakes’ and hallucinations).
the package is torn, shows signs of tampering or the films do not look quite right.
Check with your doctor if you:
have any other medical conditions such as asthma or other breathing problems, thyroid
problems, prostate problems, problems with excess alcohol use, problems with drowsiness,
Adrenal gland problems (e.g. Addison’s disease), Kyphoscoliosis (hunchback disease),
low blood pressure, urination problems, kidney problems, liver problems,
have head injuries or have a condition where you have increased pressure within your
head
have problems related to the biliary tract
have a history of seizures or if you have severe mental problems or hallucinations
(seeing or hearing things that are not really there).
take any medicines for any other condition.
Some people have died from respiratory failure (inability to breathe) when using benzodiazepines
(medicines used to treat anxiety or sleeping problems) at the same time as SUBUTEX.
SUBUTEX can cause withdrawal symptoms (dependence) if you
take it less than six hours after you use a short acting opioid (such as morphine
or heroin) or less than 24 hours after a long-acting opioid (such as morphine or heroin)
or less than 24 hours after a long-acting opioid (such as methadone) when taken sublingually
or buccally
stop using the medicine too quickly. Withdrawal symptoms may be delayed in some cases.
SUBUTEX may cause
fatal respiratory failure in children who accidentally ingest it.
drowsiness, which may be made worse if you also drink alcohol or take sedatives or
anti-anxiety medicines. If you are drowsy, do not drive or operate machinery.
your blood pressure to drop suddenly, causing you to feel dizzy if you get up too
quickly from sitting or lying down.
withdrawal symptoms if taken while still under the influence of another opioid.
SUBUTEX is not intended for occasional use and should be taken only as prescribed.
Athletes should be aware that this medicine may cause a positive reaction to "anti-doping"
tests.
The safety and effectiveness in patients over 65 years of age have not been established.
Your doctor may ask you to have additional blood tests to see if this medication is
right for you.
During treatment, you may be at risk of developing certain side effects. It is important
you understand these risks and how to monitor for them. See additional information
under Section
6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
Use of SUBUTEX or other opioids by the mother during pregnancy may result in withdrawal
symptoms in the baby following birth, this is called Neonatal Abstinence Syndrome.
Neonatal Abstinence Syndrome is a condition that includes disturbances to a newborn
baby's nervous, gastro-intestinal and breathing systems. Not all babies who are exposed
to SUBUTEX in this way will have withdrawal symptoms. Talk to your doctor if you become
pregnant or plan to become pregnant during treatment with SUBUTEX. Your doctor will
help you consider the risks and benefits of continued treatment and plan for monitoring
your baby following birth. Due to the long duration of buprenorphine effect, your
baby will be monitored for several days at the end of pregnancy for effects on breathing
and for withdrawal symptoms.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any
medicines, vitamins or supplements that you buy without a prescription from your pharmacy,
supermarket or health food shop.
Some medicines may interfere with SUBUTEX and affect how it works.
In particular, tell your doctor, nurse or pharmacist if you are taking any of the
following medicines:
medicines containing alcohol
certain medicines for treating HIV/AIDS
certain medicines for treating fungal and bacterial infections
strong painkillers
cough medicines containing opioid-related substances
certain antidepressants including monoamine oxidase inhibitors
certain medicines used to treat fits or epilepsy (anti-convulsants)
sedating antihistamines
sedatives
alcohol
anti-anxiety medicines
certain medicines for high blood pressure
antipsychotic medicines
naltrexone
other opioid medicines.
Tell your doctor if you are scheduled to have surgery using a general anesthetic.
Alcohol and certain other medicines (as listed above) may increase the sedative effects
of buprenorphine which can make driving and operating machinery hazardous.
Some people have died when using sedatives (benzodiazepines) or other depressants,
alcohol or other opioids at the same time as SUBUTEX. You should not use benzodiazepines
(medicines used to treat anxiety or sleep disorders) whilst you are taking SUBUTEX
unless they are prescribed by your doctor.
Do not drink alcohol or take medicines that contain alcohol whilst you are being treated
with SUBUTEX.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins
or supplements you are taking and if these affect SUBUTEX.
4. How do I use SUBUTEX?
How much to use
Each SUBUTEX contains buprenorphine and is available in three different strengths,
0.4 mg, 2 mg and 8 mg. Your doctor will tell you how much SUBUTEX to take and you
should always follow medical advice.
On the first day the usual starting dose is 4-8 mg SUBUTEX with an additional 4 mg depending on your
needs as determined by your treating doctor.
For patients who are still using short acting opioids such as heroin, morphine, oxycodone
or codeine: when starting treatment, the dose of SUBUTEX should be taken when the first signs
of craving appear or at least 6 hours after your last use of opioid or when the first
signs of craving appear.
For patients receiving methadone: before beginning treatment with SUBUTEX, your doctor will probably reduce your dose
of methadone to the minimum daily dose that you can tolerate. The first dose of SUBUTEX
should preferably be taken at least 24 hours after your last dose of methadone or
when the first signs of craving appear.
How to use SUBUTEX
The tablets are taken sublingually. This means that you place the tablet under your
tongue and allow it to dissolve, which may take between 2 and 10 minutes. This is
the only way the tablets should be taken.
SUBUTEX is packed in child resistant blisters. Below are the instructions on how to
open the blisters.
How to remove the tablets form the blister pack
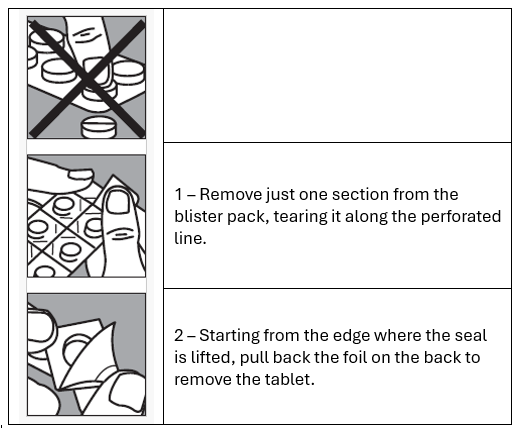
DO NOT swallow, consume food or drink until the tablet is completely dissolved. The
tablets will not work if you chew of swallow them whole. They are not designed to
be split or broken.
During your treatment, your doctor may increase your dose of SUBUTEX up to a maximum
daily dose of 32 mg, depending upon your response to treatment.
After a period of successful treatment, your doctor may gradually reduce your dose.
Depending on your condition, your dose may continue to be reduced under careful medical
supervision, until it is stopped altogether.
Do not inject SUBUTEX. Patients have died from injecting this medicine. Additionally,
when injecting SUBUTEX and also taking benzodiazepines (medicines used to treat anxiety
or sleeping problems), people were even more likely to die.
If you forget to use SUBUTEX
If you miss your dose of SUBUTEX take it as soon as you remember. If you are unsure
consult your doctor, nurse or pharmacist.
If you use too much SUBUTEX
If you think that you have used too much SUBUTEX, you may need urgent medical attention.
You should immediately:
phone the Poisons Information Centre
(by calling
13 11 26), or
contact your doctor, or
go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
Symptoms of an overdose with SUBUTEX may include:
slow, unusual or difficult breathing Slow or weak heartbeat
nausea or vomiting.
Other signs of overdose can also include problems with the 'nervous system' caused
by damage to the white matter of the brain (known as toxic leukoencephalopathy).
When seeking medical attention, take this leaflet and remaining medicine with you
to show the doctor. Also tell them about any other medicines or alcohol which have
been taken.
5. What should I know while using SUBUTEX?
Things you should do
If you are about to start on any new medicine, remind your doctor, nurse and pharmacist
who treat you that you are using or will be starting SUBUTEX.
If you are going to have surgery, tell the surgeon or anaesthetist that you are using
SUBUTEX.
It may affect other medicines used during surgery.
Keep all of your doctor/nurse/pharmacist's appointments so that your progress can
be checked.
Call your doctor straight away if you:
experience allergic reactions such as swelling of the face, lips, mouth or throat,
or severe difficulty in breathing, or
experience severe liver problems such as intense fatigue, no appetite, your skin and
eyes look yellow, or you have light coloured bowel motions or dark coloured urine.
experience severe upper abdominal pain possibly radiating to the back, nausea, vomiting
or fever as this could be symptoms associated with inflammation of the pancreas (pancreatitis)
and the biliary tract system.
have difficulty swallowing, regurgitation, chest pain that is not related to your
heart, especially if you have been using this medicine for a long time.
Remind any doctor, dentist, nurse or pharmacist you visit that you are using SUBUTEX.
Things you should not do
Do not inject SUBUTEX. Patients have died from injecting this medicine.
Do not swallow or consume food or drink until the tablet is completely dissolved.
Do not split or break the tablet. The tablet will not work if you chew or swallow
them whole.
Do not stop using this medicine suddenly, as this may cause withdrawal symptoms.
Do not take your medicine to treat any condition other than the one prescribed for
by your doctor.
Do not give your medicine to anyone else, even if they have the same condition as
you. It may harm them.
Things to be careful of
SUBUTEX may cause your blood pressure to drop suddenly, causing you to feel dizzy
if you get up too quickly from sitting or lying down.
if you feel light-headed, dizzy or faint when getting out of bed or standing up, get
up slowly.
if you stop using SUBUTEX and if you start using opioids again, your sensitivity to
opioids may change which could be dangerous. You should talk to your doctor before
you start using opioids again.
SUBUTEX contains a narcotic that can be a target for people who abuse prescription
medicines or street drugs. Therefore, keep your medications in a safe place to protect
them from theft. Never give them to anyone else.
In the event of an emergency
It is important that your family members or friends tell hospital or ambulance staff
that you are dependent on opioids (narcotics) and are being treated with SUBUTEX.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how SUBUTEX
affects you.
SUBUTEX may cause drowsiness, which may be made worse if you also drink alcohol or
take sedatives or anti-anxiety medicines. If you are drowsy, do not drive or operate
machinery.
Drinking alcohol
Do not drink alcohol or take medicines that contain alcohol whilst you are being treated
with SUBUTEX.
Tell your doctor if you drink alcohol.
Looking after your medicine
Keep the tablets in a dry place where the temperature stays below 30 °C in the original
package.
Store this medicine securely, where other people cannot access it. It may harm people
who take this medicine by accident, or intentionally when it has not been prescribed
for them.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do
not store it:
in the bathroom or near a sink, or
in the car or on window sills.
Keep it where young children cannot reach it.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy
for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of
them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you
have any further questions about side effects.
Less serious side effects
Serious side effects
Tell your doctor or pharmacist if you notice anything else that may be making you
feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can
report side effects to the Therapeutic Goods Administration online at
www.tga.gov.au/reporting-problems .
For adverse event reporting please contact:
Indivior Pty Ltd
Tel: +800-270-81901
By reporting side effects, you can help provide more information on the safety of
this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop
taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What SUBUTEX contains
|
Active ingredient
(main ingredient)
|
buprenorphine hydrochloride
|
|
Other ingredients
(inactive ingredients)
|
citric acid
lactose monohydrate
magnesium stearate
maize starch
mannitol
povidone
sodium citrate dihydrate
|
|
Potential allergens
|
lactose monohydrate and mannitol
|
Do not take this medicine if you are allergic to any of these ingredients.
What SUBUTEX looks like
SUBUTEX are white, flat oval shaped tablets and are available in the following presentations:
0.4 mg of buprenorphine (as hydrochloride). Tablets are debossed on one side with
"04" and supplied in a
blister pack (AUST R 76661)
jar/can (AUST R 76773)*.
2 mg of buprenorphine (as hydrochloride). Tablets are debossed on one side with "B2"
and supplied in a
blister pack (AUST R 76662)
jar/can (AUST R 76774)*.
8 mg of buprenorphine (as hydrochloride). Tablets are debossed on one side with "B8"
and supplied in a
blister pack (AUST R 76663)
a jar/can (AUST R 76665)*.
SUBUTEX is packed in
Aluminium/Aluminium blister packs of 7 or 28* sublingual tablets and
jars of 100* sublingual tablets*.
*Not currently supplied.
Who distributes SUBUTEX
SUBUTEX is distributed in Australia by:
Indivior Pty Ltd
78 Waterloo Road
Macquarie Park NSW 2113
Australia
This leaflet was prepared in October 2025.
® Registered Trade Mark. The trade marks mentioned in this material are the property
of their respective owners