2. What should I know before I receive VELCADE?
Do not use if you have ever had an allergic reaction to bortezomib, boron, mannitol
or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines,
or are pregnant or plan to become pregnant or are breastfeeding.
3. What if I am taking other medicines?
Some medicines may interfere with VELCADE and affect how it works.
4. How do I receive VELCADE?
Your doctor will decide what dose you will receive. The dose will be calculated from
your height and weight, as well as factors such as kidney function, liver function
and other medicines you are being given.
The doctor will determine the number of cycles of treatment also.
5. What should I know while receiving VELCADE?
|
Things you should do
|
Remind any doctor, dentist or pharmacist you visit that you are being treated with
VELCADE.
Keep all your doctor's appointments and follow your doctor's instructions.
You may need to take other medicines to help prevent unwanted side effects.
VELCADE can lower the number of white blood cells and platelets in your blood. This
means that you have an increased chance of getting an infection or bleeding. Avoid
contact with infected individuals and be careful with objects (such as razors) that
may predispose you to bleeding
|
|
Driving or using machines
|
VELCADE may cause tiredness, light-headedness, dizziness, fainting, double or blurred
vision in some people. Wait until you know how VELCADE affects you before you drive
or use any machines or tools.
|
6. Are there any side effects?
Like all medicines, VELCADE can cause side effects, although not everybody gets them.
Your doctor will discuss potential side effects with you and will explain the risks
and benefits of your treatment.
Some side effects can be serious, and you may require urgent medical attention.
Active ingredient(s):
bortezomib
Full Consumer Medicine Information (CMI)
This leaflet provides important information about using VELCADE. You should also speak to your doctor or pharmacist if you would like further information
or if you have any concerns or questions about using VELCADE.
Where to find information in this leaflet:
1. Why am I receiving VELCADE?
VELCADE contains the active ingredient bortezomib. VELCADE belongs to a group of medicines called antineoplastic or cytotoxic medicines.
You may also hear of these being called chemotherapy medicines. These medicines are
used to kill cancer cells.
VELCADE is used to treat adults with multiple myeloma (cancer of the bone marrow).
It is prescribed for patients who have not been previously treated for multiple myeloma.
It is also prescribed for patients who have received one or more prior treatments
and whose cancer is still progressing.
VELCADE is also used for the treatment of mantle cell lymphoma (a type of cancer affecting
the lymph nodes) in adults in combination with the medicines rituximab, cyclophosphamide,
doxorubicin and prednisone, for patients whose disease has not been previously treated.
2. What should I know before I receive VELCADE?
Warnings
Do not use VELCADE if:
you are allergic to bortezomib, boron, mannitol, or any of the ingredients listed
at the end of this leaflet.
Always check the ingredients to make sure you can use this medicine.
Check with your doctor if you:
have any other medical conditions especially the following:
blood disorder with a low level of red or white blood cells or platelets. This disorder
may become worse during treatment with VELCADE.
if you are suffering from diarrhoea or vomiting as this may become worse during treatment
with VELCADE.
a history of fainting, dizziness or light-headedness.
kidney problems
liver problems, including hepatitis infection.
problems with numbness, tingling or pain in the hands or feet (neuropathy). This
effect may be worsened by treatment with VELCADE.
seizures
any bleeding problems
problems with your heart
lung or breathing problems
take any medicines for any other condition.
During treatment, you may be at risk of developing certain side effects. It is important
you understand these risks and how to monitor them. See additional information under
Section
6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant. Like most
medicines used to treat cancer, VELCADE is not recommended for use during pregnancy.
Tell your doctor if you are trying to make your partner pregnant. Both men and women
receiving VELCADE and their partners must use a reliable method of contraception during
and for 3 months after receiving VELCADE.
Talk to your doctor if you are breastfeeding or intend to breastfeed. It is not known
whether VELCADE passes into breast milk. Therefore, there is a possibility that the
breast-fed baby may be affected. If you wish to restart breast-feeding after your
VELCADE treatment, you must discuss this with your doctor or nurse, who will tell
you when it is safe to do so.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any
medicines, vitamins or supplements that you buy without a prescription from your pharmacy,
supermarket or health food shop.
Some medicines may interfere with VELCADE and affect how it works.
In particular, tell your doctor if you are taking any of the following:
amiodarone, a medicine used to treat irregular heart beat
medicines used to treat viral infections such as flu, herpes and HIV
isoniazid, a medicine used to treat tuberculosis
nitrofurantoin, a medicine used to treat urinary tract infections
ketoconazole, a medicine used to treat fungal infections
ritonavir, a medicine used to treat HIV infection
rifampicin, a medicine used to treat infections such as tuberculosis
medicines used to treat high cholesterol levels in the blood
medicines used to treat diabetes
medicines that may lower blood pressure
medicine used to treat epilepsy such as carbamazepine and phenobarbital
phenytoin, a medicine used in preventing seizures
St John's Wort (Hypericum perforatum)
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins
or supplements you are taking and if these affect VELCADE.
4. How do I receive VELCADE?
Overall treatment with VELCADE must be done under the supervision of a doctor. Your
treatment with VELCADE may be given by a healthcare professional (e.g., doctor or
nurse) experienced in the administration of oncology medicines.
How much is given
Your doctor will decide what dose you will receive.
The dose will be calculated from your height and weight. It will also depend on factors
such as kidney function, liver function and other medicines you are being given.
The usual starting dose is 1.3 milligrams per square meter body surface area.
Your doctor may change the dose during treatment depending on your response
How it is given
VELCADE will be dissolved in sterile normal sodium chloride (salt) solution for injection.
The solution is given as an injection into your vein (intravenously) over 3 to 5 seconds.
The injection tube will be rinsed with a small quantity of sterile normal sodium chloride
(salt) solution.
The solution can also be given subcutaneously as an injection into your thighs (right
or left), or abdomen (right or left).
VELCADE must be given intravenously or subcutaneously only.
VELCADE must not be given into the space around the spinal cord (intrathecally).
When it is given
Multiple Myeloma
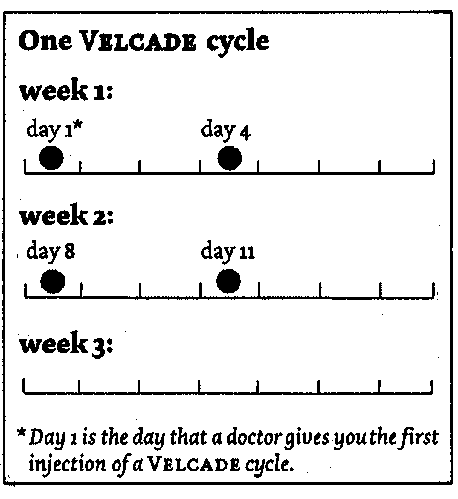
One cycle of treatment with VELCADE may consist of a total of 4 doses given over 3
weeks. Doses are given on days 1, 4, 8 and 11 followed by a ten day break from the
treatment.
When VELCADE is given with thalidomide and dexamethasone, the treatment consists of
a total of 3 cycles (9 weeks) for the induction stage. During the induction stage,
VELCADE is administered twice weekly (days 1, 4, 8 and 11).
When VELCADE is given with dexamethasone, the treatment consists of a total of 4 cycles
(12 weeks). VELCADE will be administered twice weekly (days 1, 4, 8 and 11).
When VELCADE is given with melphalan and prednisone, one cycle of treatment is 6 weeks
and the treatment consists of a total of 9 cycles (54 weeks). In Cycles 1-4, VELCADE
is administered twice weekly (days 1, 4, 8, 11, 22, 25, 29 and 32). In Cycles 5-9,
VELCADE is administered once weekly (days (1, 8, 22 and 29).
Mantle Cell Lymphoma
When VELCADE is given with rituximab, cyclophosphamide, doxorubicin and prednisone,
one cycle is 3 weeks and the treatment consists of a total of up to 8 cycles (24 weeks).
For each cycle, VELCADE is given on days 1, 4, 8 and 11, followed by a ten day break
from the treatment.
Your doctor will decide on the number of cycles of VELCADE needed. This will depend
on how you respond to treatment.
If you miss a dose of VELCADE
If you miss a dose of VELCADE, call your doctor right away to reschedule your appointment.
It is important that you do not miss a dose of this medicine.
If you are given too much VELCADE
As VELCADE is given to you under the supervision of your doctor, it is very unlikely
that you will receive too much.
However, if you experience any side effects after being given VELCADE, tell your doctor
or nurse immediately or go to Accident and Emergency at your nearest hospital.
You may need urgent medical attention.
5. What should I know while receiving VELCADE?
Things you should do
Be sure to keep all your doctor's appointments so your progress can be checked. Your doctor will want to do some blood, urine and other tests from time to time to
check on your progress and detect any unwanted side effects.
Keep follow up appointments with your doctor.
It is important to have your follow-up doses of VELCADE at the appropriate times to
get the best effects from your treatment.
Be sure to follow your doctor's instructions about other medicines you should take,
and other things you should do. You may need to take other medicines to help prevent unwanted effects of VELCADE.
You may also need to drink extra fluids if you experience vomiting and/or diarrhoea.
If you plan to have surgery, tell your doctor or dentist that you are having VELCADE.
Call your doctor straight away if you:
become pregnant or your partner becomes pregnant while being given VELCADE.
Remind any doctor, dentist or pharmacist you visit that you are using VELCADE.
Infection and bleeding risk
VELCADE can lower the number of white blood cells and platelets in your blood. This
means that you have an increased chance of getting an infection or bleeding. The
following precautions should be taken to reduce your risk of infection or bleeding:
Avoid people who have infections. Check with your doctor immediately if you think
you may be getting an infection, or if you get a fever, chills, cough, hoarse throat,
lower back or side pain or find it’s painful or difficult to urinate.
Be careful when using a toothbrush, toothpick or dental floss. Your doctor, dentist,
nurse or pharmacist may recommend other ways to clean your teeth and gums. Check
with your doctor before having any dental work.
Be careful not to cut yourself when you are using sharp objects such as a razor or
nail cutters.
Driving or using machines
Wait until you know how VELCADE affects you before you drive or use any machines or
tools.
VELCADE may cause tiredness, light-headedness, dizziness, fainting, double or blurred
vision in some people. Make sure you know how you react to VELCADE before you drive
a car, operate machinery, or do anything else that could be dangerous if you are dizzy,
lightheaded or have double or blurred vision.
You may feel dizzy or faint when you get up quickly after sitting or lying down. Getting
up slowly may help.
Drinking alcohol
Tell your doctor if you drink alcohol. If you drink alcohol, dizziness or light-headedness may be worse.
Looking after your medicine
VELCADE is usually stored in the hospital, clinic or at the pharmacy.
Your doctor, pharmacist or nurse is responsible for storing this medicine and disposing
of any unused product correctly.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of
them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you
have any further questions about side effects.
Less serious side effects
Serious side effects
Tell your doctor or pharmacist if you notice anything else that may be making you
feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can
report side effects to the Therapeutic Goods Administration online at
www.tga.gov.au/reporting-problems . By reporting side effects, you can help provide more information on the safety of
this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop
taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What VELCADE contains
|
Active ingredient
(main ingredient)
|
bortezomib
|
|
Other ingredients
(inactive ingredients)
|
mannitol (E 421)
nitrogen
|
Do not take this medicine if you are allergic to any of these ingredients.
What VELCADE looks like
VELCADE is a white to off-white powder in a glass vial. Each pack contains one single-use
vial:
VELCADE 3.5 mg vial (AUST R 104542)
VELCADE 3 mg vial (AUST R 238257)
VELCADE 1 mg vial (AUST R 148329)
Not all presentations may be supplied.
Before injection, VELCADE powder is dissolved in a small quantity of sterile, sodium
chloride solution. The solution for injection is clear and colourless.
Who distributes VELCADE
JANSSEN-CILAG Pty Ltd
17 Khartoum Rd
Macquarie Park NSW 2113 Australia
Telephone: 1800 226 334
This leaflet was prepared in December 2025.