Over the course of the last few years, nanomedicine research has grown rapidly, with an enhanced focus on drug delivery.
A number of benefits are offered by nanoparticles – such as reduced toxicity, minimum side effects, and targeted efficacy. Having said that, it becomes extremely essential to control the size of these nanoparticles.
Despite the fact that a large majority of the particle size measurements are performed in the laboratory, these measurements can also be performed at-line in the production setting. The following article discusses BIND Therapeutics’ ground-breaking work in Cambridge, Massachusetts to implement online dynamic light scattering (DLS) measurements in the nanoparticle drug candidate Accurins’ production process.
Accurins
BIND Therapeutics is a biopharmaceutical company specializing in the development of targeted nanoparticle technologies known as Accurins (Figure 1). The drug Accurins is utilized in the treatment of serious diseases such as cancer. Today, the company develops a nanotechnology-enabled platform, through the integration of controlled release polymer systems, which target and deliver large payloads of therapeutic agents. Thus, this platform will thereby lead to a new class of targeted therapeutics.
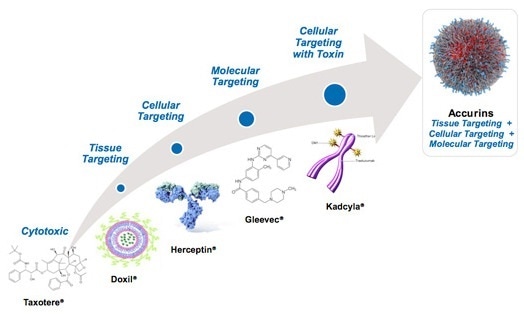
Figure 1. BIND Accurins technology
Containing polylactide - polyethylene glycol (PLA-PEG) co-polymers, Accurins contains an active pharmaceutical ingredient (API) core. What’s more, the PLA portion of the co-polymers is biodegradable – which offers a moderately hydrophobic core for encapsulating hydrophobic APIs. In addition, the portion of the polymer containing hydrophilic PEG is anticipated to coat the particles’ surface and enable an escape from opsonization. This ultimately prevents their removal from blood circulation by the phagocytic cells of the reticuloendothelial system (RES).
Accurins are produced through a nanoemulsion process. In other words, the nanoemulsion process utilizes high pressure homogenization to shear organic droplets spread within an immiscible aqueous phase. In order to measure the final size distribution of the drug product, thus it becomes vital to control the size of droplets. Nevertheless, a host of different factors affect the droplet size. These include particle formulation, raw material attributes, aqueous phase composition, homogenizer mechanical properties, as well as process parameters. Finally, one of the process parameters is homogenizer pressure – i.e., a factor that can be easily manipulated to change size after the batch has been produced.
At present, BIND is developing BIND-014 – an Accurin that will deliver docetaxel to cancer and tumor cells that express prostate- specific membrane antigen (PSMA). This article describes experiments for BIND-014 Accurins.
At-Line Dynamic Light Scattering
The technique used to determine the size of submicron particles is known as Dynamic light scattering (DLS) . For decades, this method has been successfully used in labs. However, until today, few in-process solutions exist.
Based on the principle that tiny particles move arbitrarily in fluids, DLS does so by utilizing Brownian motion. Equation 1 depicts how the system detects the translational diffusion caused by Brownian motion, and further utilizes this diffusion to solve the Stokes-Einstein equation to measure the particle size.
D = kBT/6πηR (1)
Where:
D = diffusion coefficient
kB = Boltzmann constant
π = viscosity
R = particle radius
A number of systems have been installed by Entegris at the client’s production operations. These are designed for the monitoring of particle size during production runs. The process involves the advanced at-line system procuring a sample from the process and then diluting it, to prevent the effects of multiple scattering. Subsequently, it then measures the sample and repeats the entire process – as seen in Figure 2. The entire measurement process takes about 2 minutes and delivers accurate and continuous particle size data to process engineers controlling the production operation.
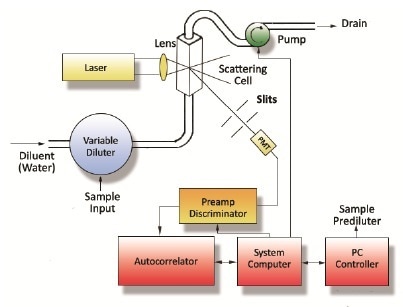
Figure 2. Simplified diagram of the at-line DLS system, with autodilution
Experimental Details
Entegris’ at-line DLS system was installed downstream of a high-pressure homogenizer. Moreover, it was configured in such a manner that it is efficiently able to capture an emulsion sample from the process stream every 2 minutes. What’s more, the DLS’ fluidics is configured so as to enable the dilution of the emulsion sample in water, a process that is similar to the downstream Accurin process. Further, this is then auto-diluted in a flow cell to a concentration to create an appropriate light scattering intensity (~300 kCt/s).
Three batches are illustrated here:
- To develop a correlation between size and pressure, the first batch is made with 11 in-process samples and inconsistent pressure all through the homogenization
- The second one is produced with slightly different process conditions, ensuring a somewhat smaller than target size for the first two in-process specimens. As soon as the pressure is adjusted, the size is restored to target for the final four samples
- Finally, a clinical-scale development batch displays steady size readings during the course of the eight samples obtained at approximately 5 minute intervals. This batch validates the suitability of the pressure set point.
Results and Discussion
As seen in Figures 3 and 4, results obtained from the initial experiment highlight the anticipated correlation between size and pressure. As observed from the trend line curve fit –the response of size to pressure is in the range of 9 nm/1,000 psig.
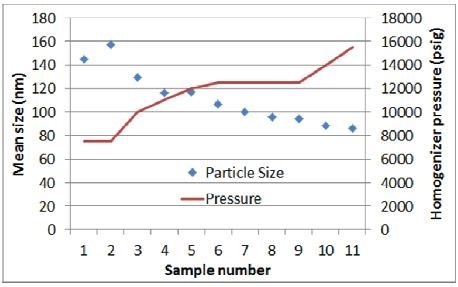
Figure 3. Homogenizer pressure vs. particle size
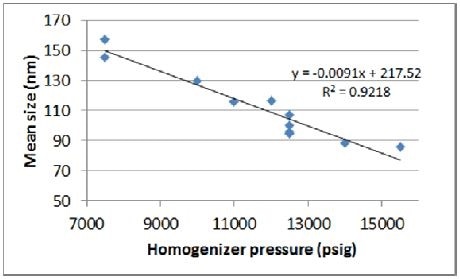
Figure 4. Correlation of pressure to mean size
As observed from the second experiment, initial size readings of approximately 5 to 7 nm were calculated under the target size. Thus, the pressure was adjusted by decreasing 1,000 psig. Later on, as predicted, the size of the mean particle increased by roughly 5 to 10 nm (Figure 5).
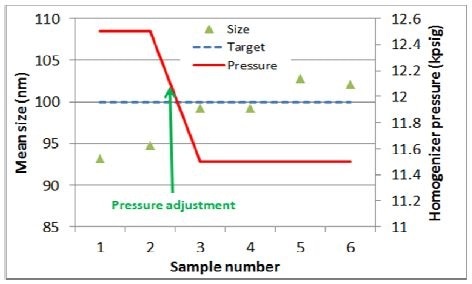
Figure 5. Homogenizer pressure vs. particle size
Finally, as seen in Figure 6, at clinical-scale (as compared to the initial experiment), the final set of data was obtained by means of the at-line sizer. While BIND ensured the availability of the required processes to alter the pressure in case the size dropped below the target range, such a situation did not arise. All eight measurements obtained were found within the 100 nm target range.
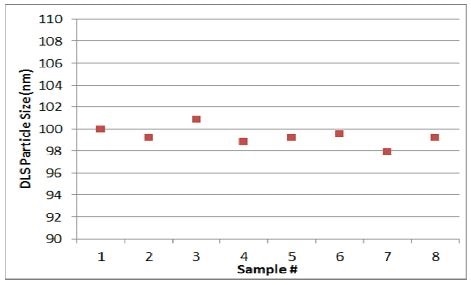
Figure 6. Mean size during a batch run
Conclusion
Incorporated in the Accurin production process, Entegris’ at-line DLS system was successfully utilized to discover optimum conditions as well as ensuring that particle size was within the preferred specification during the entire batch run.
Further, the measurements taken at-line reduced the lag time between process changes, as well as produced particle size information required for the assessment of the change in terms of the desired effect. Finally, the product quality is suitably monitored in comparison to transporting samples to laboratories for off-line batch testing.
About Entegris
Since our inception in 1978 we have been providing leading edge instrumentation for the field of particle size analysis. We are an applications driven company that provides solutions to our customer's most complex particle sizing and zeta potential monitoring problems. From wet to dry, online to research laboratory we have engineered a complete family of modular designed instruments that can be configured to meet the specific demands of an application.
Single Particle Optical Sizing (SPOS)
The Entegris AccuSizer operates using the principle of single particle optical sizing (SPOS). This technique generates high accuracy, high resolution particle size distribution results as well as concentration data in particles/mL. The AccuSizer platform includes multiple configurations designed to meet a wide array of applications including laboratory and online systems. Multiple sensor options cover a wide range of particle size and concentration limits. Advanced fluidics modules can handle low concentration samples requiring no dilution to highly concentrated inks and CMP slurries using our patented two stage exponential dilution system.
Dynamic Light Scattering
The Nicomp dynamic light scattering (DLS) system measures particle size below 1 nm and zeta potential to study particle charge. The unique Nicomp algorithm provides the highest resolution results for samples containing more than one peak. Other unique capabilities include auto-dilution and auto-samplers to facilitate high sample throughput.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.