Pharmaceutical supply chain processes, while often intricate and complex, ensure that patients receive appropriate medicines promptly and safely. Managing a challenging supply chain well is vital to ensure a pharmaceutical business runs smoothly to achieve success.
Continue reading to learn how companies in the pharmaceutical industry work with commercial supply chains and the challenges they may need to negotiate.
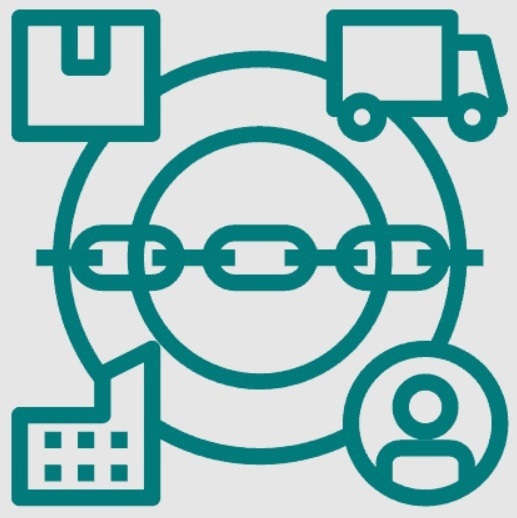
Image Credit: DSI, a PLG Company
Commercial Supply Chains in the Pharmaceutical Industry
Competition is intense across the pharmaceutical industry, and regulations are rigorous. Pharmaceutical supply chains are linked to compliance and profitability, two business metrics that depend on traceability and are often in competition regarding supply chain management. Moreover, there are a significant number of stakeholders and potential issues that must be taken into account during the process, such as manufacturers, suppliers, distributors, pharmacies, and patients.
Ensuring each part of the process and stakeholder chain is managed appropriately requires using either traceability systems, supply chain managers or a combination of both.
There are five key stages of any pharmaceutical supply chain:
- Pharmaceutical products are manufactured at the production site to meet market demand.
- Manufacturers manage the transportation of products to distributors to ensure they are promptly delivered, as they sometimes may be mistakenly delivered directly to pharmacies.
- Products are delivered by manufacturers or wholesale distributors to pharmacies, which include hospitals, web-based companies, retail pharmacies and more.
- Products are processed through pharmacy benefit managers (PBMs) and their systems. PBMs play a crucial role in consumer drug purchases despite not being directly connected to the supply chain process. PBMs manage purchases by defining how much each party will pay.
- Pharmacies dispense pharmaceutical products to patients. They then relay crucial information to stakeholders in the chain and assume the responsibility of ensuring customers know how to use the drugs safely and appropriately.
DSI Consultants Support Supply Chain Management
Having the capacity to deliver a constant supply of goods to the market is critical to preserving a business’s reputation. Organizations must be equipped to manage their clinical and commercial supply chain operations in an appropriate manner.
DS InPharmatics consultants support supply chain management and can provide strategies and implementation to ensure the business is running efficiently.
DSI’s list of services includes the following:
- CMO and CPO selection and management
- Contingency strategies
- Cost of goods evaluation
- End-to-end supply chain assessment
- Forecasting
- Inventory strategies
- Supply chain optimization
DSI provides a diverse range of services to support, manage and improve any business. With a team of expert consultants possessing decades of experience across each stage of the product development process, DSI can help cover regulatory and technical project management consulting services. If you wish to start your journey with DS InPharmatics, get in touch today.
About DS InPharmatics 
DS InPharmatics (DSI) provides regulatory, technical, and project management consulting services to healthcare product companies that manufacture and/or market pharmaceuticals, biopharmaceuticals, and cellular and gene therapy products.
Since 2007 we have provided our clients with innovative strategies and exceptional quality work products intended to enhance product development, approval, and marketing presence. Whether advocating CMC strategy, directing CMC operations or developing CMC submission content that represent the best interests of emerging biotech, we focus on the critical CMC issues and build programs that enhance development.
In April 2021 we were thrilled to announce that DSI has just become part of ProductLife Group.
French-headquartered ProductLife Group (PLG) is well-known in the Life Sciences market. It has a track record of successfully managing global outsourcing programs and insourcing services for its international client base. The company is on a mission to help transform human health outcomes by optimizing regulatory affairs, safety & vigilance, and quality compliance for life sciences organizations worldwide.
The fit between our two organizations could not be more perfect. We will complement PLG's growing biotech services portfolio. US biotech sponsors recognize DSI as a leader in consulting for go-to-market strategies and RA pre-market consulting. At the same time, PLG has a strong reputation for managing end-to-end outsourcing of regulatory affairs and pharmacovigilance activities worldwide.
Our merger with PLG will harness our combined strengths, offering our clients on both sides of the Atlantic support with their developed drugs approvals and post-approvals compliance, plus advisory services on the best market strategies to deliver a rapid ROI on their development. Together we will offer our clients increased pharmacovigilance capabilities - including a QPPV; pharmacovigilance consulting; and a fully validated safety database - as well as complementary toxicology-related services; RIM/electronic document management services; and support for medical device regulatory requirements.
We see enormous potential in this new chapter for DSI and you, our clients. As a PLG company, we have the opportunity to become part of a global force in life sciences regulatory and compliance solutions and services, and we're incredibly excited to add our momentum to that effort.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.