Cancer is characterized by the abnormal growth of cells that spread from their original location to other parts of the body. Despite extensive research efforts, a cure for the disease has yet to be found, largely due to its heterogeneous nature.
Cancer is a complex and diverse disease that can originate in various parts of the body and exhibit varying symptoms. It can be challenging to diagnose and treat due to its many forms. The National Cancer Institute recognizes over 100 distinct types of cancers, each requiring specific screening methods, diagnostic tests, and treatment approaches.3
What are the barriers to curing cancer?
The obstacles faced by cancer researchers and clinicians in delivering effective treatments and quality care to patients are abundant and complex. The complexities of diagnosis, treatment, and quality of life create countless variables in cancer care.
The heterogeneity of cancer presents the biggest challenge to finding a cure, making diagnosis complex, treatment choices complicated, and prognosis uncertain.4,5 This diversity also means that a single treatment cannot effectively target all cancers.
Early detection is the key. Early detection significantly improves the 5-year survival rate for most cancers in the United States, with rates exceeding 90%.6
Yet, current cancer detection strategies are only able to find approximately 29% of tumors, mainly relying on patient screening approaches like imaging and symptom reporting, which are frequently used after cancer has spread to other organs. These methods are pricey and unreliable.7
Genomic research is currently a major focus in the study of cancer. Although genomes research still holds out enormous potential and much has been learned about how different tumors originate and metastasize thanks to genomics, additional strategies are now becoming accessible that can make a big difference.
An early 20th-century discovery, the stimulation of autoantibody production by tumors, is seeing renewed attention.8 Although genetics may suggest potential, the presence of an autoantibody signature indicates the actual occurrence.
Perhaps most significantly, autoantibodies serve as an early indicator of cancer, long before it has spread, as a result of ongoing immune surveillance processes that track changes in the host proteome associated with the disease.
These immunoglobulins not only aid in early cancer detection but also provide opportunities for patient stratification and shed light on therapy resistance and potential therapeutic targets.9, 10
What is the relationship between cancer and autoantibodies?
In the early stages of tumor growth, cancer cells can trigger an immune response and start the production of autoantibodies (Figure 1).11-13 Like any other cell, cancer cells produce proteins and use energy to support their growth.
Some expressed proteins are novel and cancer-cell specific, known as tumor-specific antigens (TSA). Others are host proteins with altered expression, called tumor-associated antigens (TAA). TSA results from mutated or variant genes or other abnormal expressions or modifications in cancer cells.
Tumor-associated antigens are self-proteins with abnormal expression, either due to production in the wrong location or expression at an incorrect level. The immune system recognizes this novel and potentially harmful expression and generates antibodies, leading to the production of autoantibodies that signify the disease.11
Circulating autoantibodies identify these proteins before any existing screening method.7,10,11,13-15 They persist as long as the disease persists.
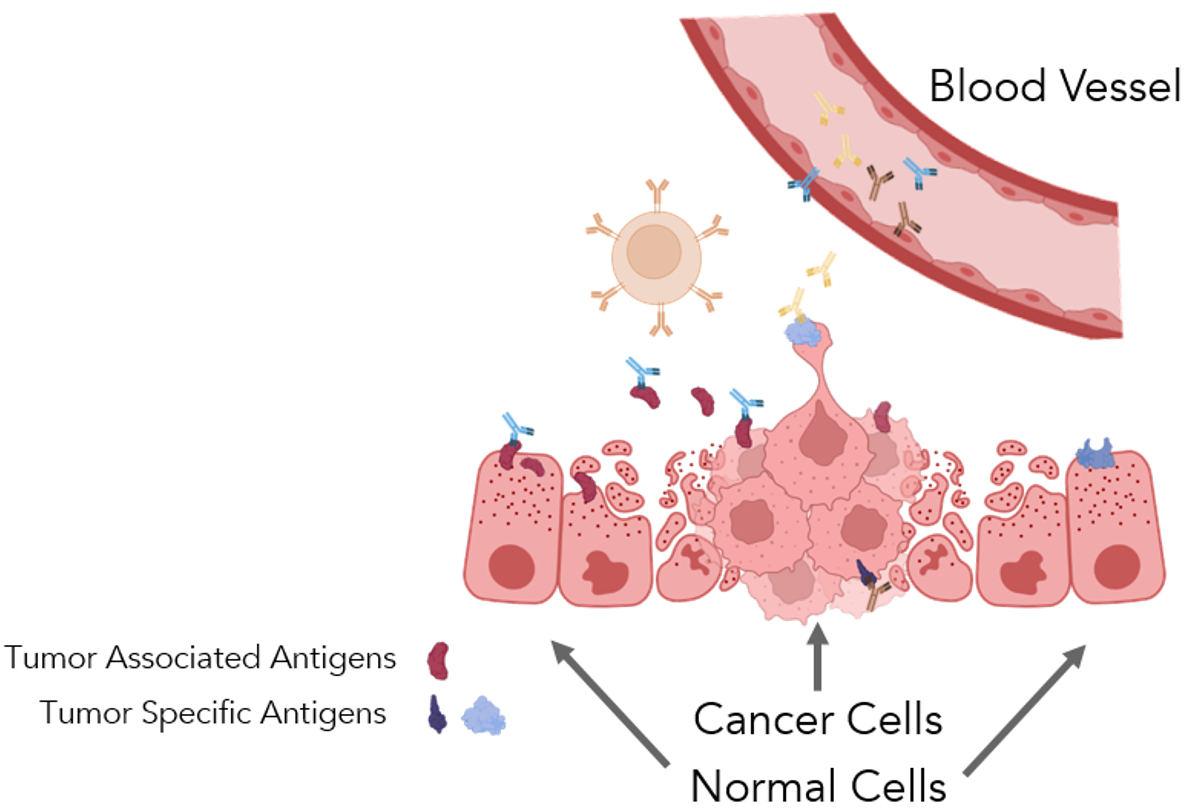
Figure 1. Tumor growth damages surrounding tissue, initiating an immune response. The antigenic proteins synthesized by cancer cells may be common to the host, tumor-associated antigens, or they may be unique, tumor-specific antigens. Autoantibodies are antibodies produced against tumor-associated and tumor-specific antigens. These are produced in detectable quantities, circulating in the blood via the Humoral Immune System. Figure created with Biorender.com
Autoantibodies, due to their early expression and persistence after the appearance of tumors, serve as ideal liquid biopsy biomarkers for cancer detection and monitoring.
What role do autoantibodies play?
Autoantibodies in cancer detection and prognosis
The diagnosis of cancer is usually made for individual patients. Only four population screening protocols are currently in use: for breast, colorectal, cervical, and lung cancers. These tests utilize imaging techniques, and the tumor must be large enough to be seen.
As there are no effective screening methods for other types of cancer, symptom reporting and biopsy are frequently used.7 Blood-based biomarkers have significant promise for early cancer diagnosis in general. Blood samples are simple to obtain and contain a wide range of quantifiable analytes.
Blood testing is a widely accepted method in the medical field, as it offers numerous benefits, including quicker results and minimal discomfort to patients, compared to tissue biopsies.16,17
While various blood-based markers for cancer already exist,18 blood testing is typically complementary to tissue biopsy, as it typically only screens for single markers, which are not often definitive on their own.
If a blood test yields a positive result, a tissue biopsy is usually the next step. The prospect of using autoantibody screening for early cancer detection is promising and has the potential to enhance the entire treatment process.
Autoantibody analysis from patient sera is a potent, less intrusive method that is ideal for early detection. A profile of autoantibodies found in a patient group can be utilized as a diagnostic tool, a prognosis indicator, and to pinpoint possible treatment targets because tumors cause several autoantibodies to be created rather than just one.9
The detection of autoantibodies to p53 in a lung cancer patient, for instance, gave medical practitioners the opportunity to take action before the emergence of the tumor.15,19
In a recent and extensive study by Patel et al. (2022), 60 promising autoantibodies were discovered from a screening of over 1600 antigens in a cohort of 157 patients diagnosed with non-small cell lung cancer (NSCLC). Eighteen of the 60 autoantibodies were found to correlate with survival rates.
An analysis of various combinations of these 18 autoantibodies revealed that 13 had a strong correlation with 5-year patient survival, as shown in Table 1. This was further validated in a separate cohort, highlighting the reliability of this approach.
Interestingly, some of these autoantibodies were found to be cancer-testis antigens.20 These are fetal antigens that are inactive in all adult somatic tissues except the testes, suggesting that patients with a poor prognosis may have a different and more cancer stem cell-like subtype of NSCLC.
Table 1. Set of autoantibodies found to strongly correlate with poor survival. Source: Sengenics
| Biomarker |
Name |
| SPATA19* |
Spermatogenesis-associated protein 19 |
| TSPY3* |
Cancer Testis Antigen 78/Testis Specific Protein Y-Linked 3 |
| GLS2 |
Glutaminase 2 |
| TCEA2* |
Transcription Elongation Factor A2/TFIIS/Testis-Specific SII gene |
| TSGA10* |
Testis-specific gene protein 10/Cancer Testis Antigen 79 |
| HMGN5 |
High Mobility Group Nucleosome Binding Domain 5/NSBP1 |
| LUZP4* |
Leucine Zipper Protein 4/Cancer Testis Antigen 28 |
| HDAC4 |
Histone Deacetylase 4 |
| SPACA3* |
Sperm Acrosome membrane-associated protein 3/Cancer Testis Antigen 54 |
| IMPDH1 |
Inosine Monophosphate Dehydrogenase 1/LCA11 |
| TXN2 |
Thioredoxin 2/MT-TRX/COXPD29 |
| TFG |
Trafficking from ER to Golgi Regulator/TRKT3 Oncogene/TRK-Fused Gene Protein |
| PPP2R1A |
Protein Phosphatase 2 Scaffold Subunit alpha/Serine Threonine Protein Phosphatase 2A |
*Cancer Testis Antigen.
Patel et al. discovered a unique profile of 13 autoantibodies with high prediction power of poor prognosis among post-operative non-small cell lung cancer patients by analyzing autoantibodies in patient samples.
None of the 13 proteins discovered by this autoantibody screen are currently included in the National Cancer Institute’s list of Tumor Markers in Common Use.18 A massive autoantibody screen was used in this study to identify a collection of indicators strongly predictive of cancer prognosis, which shows its simplicity and effectiveness.
The use of autoantibodies as predictive markers may surpass that of genetic markers. 20,21 Cancer heterogeneity leads to the production of numerous tumor-specific and tumor-associated autoantigens across different cancers, patients, and stages, which could provide a more comprehensive picture.
The presence of early-stage cancer and the patient’s prognosis can be determined by evaluating numerous autoantibodies at once to take advantage of this heterogeneity. Individual genes are often the focus of current genetic tests.
A combination of several autoantibodies forming a signature will have a greater predictive value compared to a single marker. Moreover, autoantibody panels offer fresh perspectives and potential targets for treating NSCLC.
As an example, the cancer-testis antigens (CTAs), which were prominently featured in the signature identified by Patel et al., are typically expressed in male germ cells and during embryonic development.
Therefore, their ectopic expression in NSCLC patients (male and female) may offer a great, well-targeted therapeutic target; some CTAs, such as NY-ESO-1 and MAGEA3, have been suggested as vaccine targets in malignancies like melanoma. Other prognostic panels will certainly appear on future screens.20
Autoantibodies in personalized medicine
From the mid-1990s to around 2010, the pharmaceutical industry experienced a sharp rise in drug development costs, yet fewer drugs were approved for the market.
The causes were multifaceted and included, among other things, stricter standards for superior efficacy, subpar patient reactions, and inconsistent regulations for newly created medications, which frequently led to subpar safety profiles and unsuccessful treatments.22-24
Improved decision-making, including the implementation of biomarker-driven targeted medicine, seems to have a positive impact on reversing this trend.23 Identifying patients who are most likely to benefit from medication and experience fewer side effects can enhance both safety and efficacy profiles while satisfying regulatory requirements.23-24
The use of genetic markers has enhanced drug development decision-making and patient stratification.24 Autoantibodies are in a unique position to advance precision medicine and further our cause.
Autoantibody signatures are well-suited for early detection, prognosis, patient classification, and treatment response prediction.9,10 Recent studies have started to explore the predictive capability of autoantibodies, especially in the context of immunotherapy.
New immunotherapeutic therapies have improved cancer treatment over the last 15 years. Immune checkpoint blockade, CAR-T, and anti-CTLA4 immune modulation are just a few immunotherapy-based treatment approaches that have received FDA and EMA approval.25,26
The majority of these therapies focus on non-solid malignancies like leukemia. Despite blatant achievements, 40-80 % of patients either do not respond to the medication or eventually develop resistance.27
Additionally, almost all patients report side effects at some point while receiving medication, even years later.27-29 To determine which patients are most likely to respond to immunotherapy and to foretell unfavorable immune-related events, biomarkers are required.
This will make it easier for doctors to recommend the right therapies, co-therapies, and options for follow-up care. It is interesting to note that autoantibodies have been found in patient sera after immune checkpoint inhibitor immunotherapies.29,30
Immune checkpoint inhibition (ICI) immunotherapy is a relatively new strategy, and studies regarding immune-related adverse events are ongoing.
In a Phase I dose-escalation study, Da Gamma Duarte et al.31 aimed to identify autoantibody signatures that were predictive of outcomes and adverse events in five patients with stage III/IV metastatic melanoma. The researchers quantified autoantibody titers against over 1,600 antigens.
This study had several distinctive features. First off, the study by Da Gamma Duarte looked at many more autoantibodies, although it is known that immune checkpoint inhibitors cause autoantibodies associated with classical autoimmune disease.32
The study also employed a protein microarray, which was composed of properly folded proteins. This was a crucial aspect, as most protein microarrays do not use folded proteins. Antibodies and autoantibodies require proper tertiary structure for effective binding, making this characteristic of the protein microarray particularly important.
Comparing well-folded proteins to fragmented, unfolded, or linear peptides increases specificity.1,2,33–37 Thirdly, the trial employed a cutting-edge strategy for managing advanced-stage melanoma.
The study participants underwent initial treatment with Bacillus Calmette-Guerin (BCG), followed by treatment with the immune checkpoint inhibitor Ipilimumab 36 days later. They received maintenance dosing with Ipilimumab every 12 weeks thereafter. In addition, sera from healthy donors were also collected for comparison purposes.
The researchers were able to compare autoantibody signatures in patients with various therapy outcomes to autoantibody signatures in healthy controls because of this innovative design. Serious adverse events — the trial’s intended endpoint — led to an early termination.
Further investigation is necessary to fully understand the unique autoantibody signatures linked to adverse events, given the limited sample size and premature conclusion of previous studies.
Despite the aforementioned limitations, the extensive screening of over 1600 autoantibodies conducted by the researchers resulted in the unmistakable discovery of a correlation between adverse events and elevated levels of autoantibodies.
Still, some labs have not found a connection between autoantibodies and unfavorable outcomes or cancer progression,40 while other labs have produced comparable results.29,39
It is crucial to remember that these latter studies did not collect a wide variety of autoantibodies, and they also did not base their findings on appropriately folded proteins.
Although the promising early results need additional validation in bigger cohorts,30 autoantibody detection following immune-related adverse events has increased attention on the predictive and prognostic utility of autoantibodies in immunotherapeutic treatment planning.30
Additionally, this would be improved by using a thorough, very specific technique to assess the enormous number of autoantibodies that are altered by both the illness and the treatment.
The work by De Gamma Duarte offers a road map for a thorough future investigation into autoantibody relevance in this space.31
Autoantibodies in drug discovery
It takes a comprehensive procedure that includes pathway identification, drug modeling, safety assessment, and potential efficacy to identify therapeutically feasible targets to treat cancer.
By detecting abnormal proteins that might be implicated in tumor progression, patient autoantibody fingerprints provide insight into the biology of cancer. As genetics do not always reflect the metabolism of the tumor, genetic techniques have been able to find hundreds of genomes for dozens of cancers but very few therapeutic targets.
A functional relationship between tumor biology and abnormal protein expression, including autoantigens distinguished by the presence of homologous autoantibodies, may make it easier to identify novel potential treatment targets.41,42
For instance, the creation of Caplacizumab to treat acquired thrombotic thrombocytopenic purpura (TTP), a non-cancerous condition, was dependent, in part, on the discovery of autoantibodies to aid in the identification of prospective therapeutic targets, in this case, the enzyme ADAMTS13.43
Autoantibodies may also highlight pathways that can help in the battle against cancer. The advantages of comprehending autoantibody physiology are not yet fully appreciated. Autoantibodies in healthy persons have been seen in the past,44,45 and they are not only found in autoimmune disorders and cancer.
They have thus been taken into account for illness prevention. Autoantibodies may serve positive purposes such as antibacterial, hygienic, and immunoregulatory, according to studies conducted in the 1980s.44,46
In a study that looked at samples from the 14th century in Europe, Klunk et al. (2022) revealed a distinct level of genetic selection affecting autoantibodies.
Antigen-presenting aminopeptidase, ERAP2, in a mutant form, conferred some degree of immunity against bubonic plague. Interestingly, the ERAP2 variation has been linked to Crohn’s disease.47
The implications of this discovery for the study of cancer and other infectious diseases include the possibility that autoantibodies play a part in the evolutionarily generated defense against disease. They might also draw attention to exploitable cancer development routes.
For instance, Shah et al. (2019) discovered that scleroderma patients with sera that carried autoantibodies against RNA polymerase I (anti-RPA194)48 had a decreased incidence of malignancy, suggesting that this polymerase may be a good treatment target.
While immune-related side outcomes following cancer therapies often resemble traditional autoimmune diseases, some autoimmune diseases, such as SLE and multiple myeloma, Crohn’s disease, and colorectal cancer, are increasingly recognized as risk factors for particular cancers (e.g., autoimmune hepatitis; inflammatory arthritis; cutaneous disorders).
Thus, it is important to continue researching the causal relationships between autoimmune diseases, malignancies, and immune-related adverse outcomes.
Conclusions
Autoantibodies were first disregarded when they were first identified since they were thought to be signs of sickness or tissue damage and not the immune system’s usual function.8 However, it was already clear in these early stages that autoantibodies had significant diagnostic potential.
The distinctive autoantibody production patterns reflect the highly diverse character of cancer. Cancer is heterogeneous, which implies that the same tumor in various patients may develop at various rates, metastasize in various ways, cause various symptoms, and so on.
Cancerous cells use a variety of metabolic pathways to carry out these functions, and as a result, they produce more ectopically produced and aberrant proteins than healthy tissue, giving them away to an immune system scanning the environment for anything unusual.
In the past, researchers and doctors have searched among these pathways for a single, potent target that can stop or eradicate cancer. For instance, targets that may control transcription or proliferation (such as paclitaxel and trabectedin) (doxorubicin). These medications are recommended just for particular cancer types and are used judiciously.
As a result, although cancer is varied, cancer treatment has not been. A collection of proteins specific to the cancer are represented by autoantibody signatures.
Using a high-fidelity expression system, high throughput processing, potent machine-learning-based bioinformatics algorithms, and appropriately folded proteins are essential for efficiently finding predictive autoantibodies.
Researchers can analyze hundreds or thousands of proteins using high-density microarrays. Machine learning streamlines the analysis of different autoantibody permutations while taking into account particular patient factors, which ultimately results in highly specific, predictive biomarkers.
SIDEBAR 1: How KREX protein folding technology works
The creation of properly folded proteins represents a significant advancement in autoantibody screening techniques.1,2 High specificity and proper antibody/antigen binding are supported by the use of full-length, well-folded proteins.
KREX technology is used in Sengenics microarrays to guarantee that the array proteins are functional, full-length, and correctly folded.
KREX, which stands for correctly folded recombinantly expressed protein, refers to a patented method by Sengenics for expressing and immobilizing correctly folded, fully functional proteins on a surface coated with streptavidin while using the protein biotin carboxyl carrier protein (BCCP) as a folding marker.
As a result of protein misfolding or fragmentation, BCCP is unable to attach to the streptavidin-coated surface because its biotinylation site is unavailable. Misfolded proteins are simply washed away.
This method was created to achieve exceptional signal-to-noise for fine detection of autoantibody signatures while maintaining conformational epitopes and ensuring excellent antibody-epitope interaction.
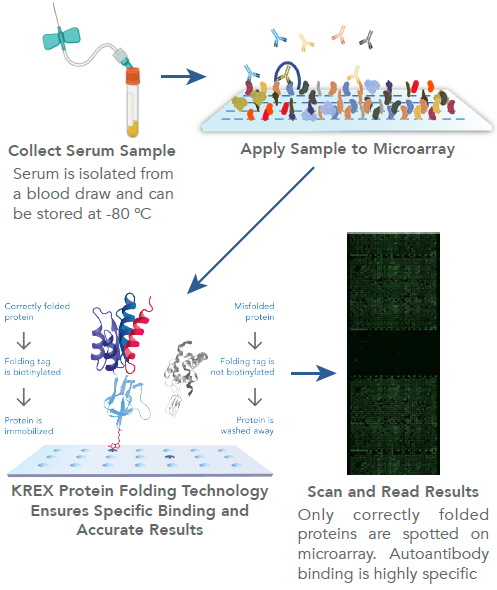
Image Credit: Sengenics
SIDEBAR 2: How are autoantibodies measured?
Autoantibodies were initially linked to disease and cancer over a century ago.8,49-52 The original method of detecting autoantibodies was through complement fixation, in which the presence of autoantibodies in patient sera led to erythrocyte cell lysis.
The origin of immunodiagnosis can be traced back to research efforts;14 however, the use of complement fixation was not a suitable approach for biomarker discovery. It was not until the mid-2000s that technology emerged that was capable of providing high-throughput and highly specific results for autoantibodies.1,2,36,37,53
In the late 1990s, gene microarrays were leading in understanding expressed genes, but applying this to proteins was a challenge. The human genome has around 20K genes, but the proteome is thought to have more than 1 million proteoforms, making the process difficult.33
Labeling, capturing, and analyzing this large library takes time, effort, and significant computing power.

Figure 2. A sample-labeled Sengenics i-Ome Microarray Chip. Image Credit: Sengenics
For detecting antibodies, proteins or peptides are usually attached to a stationary surface, such as a glass slide, in a fixed pattern.
A single slide can have thousands of proteins or peptides attached, allowing the detection of thousands of different antibodies from a limited sample of patient serum. Indirect immunofluorescence is commonly used for sample labeling and identification.33,36,54
For detecting autoantibodies, the microarray is incubated with patient sera that contain autoantibodies that bind to their corresponding epitopes. The labeling process is finished by adding a secondary antibody that is fluorescently labeled (Figure 2).
Accurate encoding of the autoantigen and preservation of its shape is crucial, as any deviation can result in loss of specific binding.33-35 Commercial protein microarrays typically produce proteins and peptides recombinantly through the use of expression vectors.
The design, expression system, vector, protein adhesion process, surface chemistry, and tertiary structure of the protein all impact the accuracy of antibody detection results.36 The KREX technology utilized with the i-Ome protein microarray effectively addresses these variables (see Sidebar 1).
References
- Matsuoka, K., et al., Simple screening method for autoantigen proteins using the N-terminal biotinylated protein library produced by wheat cell-free synthesis. J Proteome Res, 2010. 9(8): p. 4264-73.
- Beeton-Kempen, N., et al., Development of a novel, quantitative protein microarray platform for the multiplexed serological analysis of autoantibodies to cancer-testis antigens. Int J Cancer, 2014. 135(8): p. 1842-51.
- Cancer Types. 2019 [cited 2022 10/31/2022]; Available from: https://www.cancer.gov/types.
- Hinohara, K. and K. Polyak, Intratumoral Heterogeneity: More Than Just Mutations. Trends Cell Biol, 2019. 29(7): p. 569-579.
- Robertson-Tessi, M. and A.R. Anderson, Big Bang and context-driven collapse. Nat Genet, 2015. 47(3): p. 196-7.
- UnitedStatesCancerStatistics. Cancer. 2020 [cited 2020; Available from: Www.cdc.gov. https://www.cdc.gov/cancer/uscs/.
- Hackshaw, A., et al., Estimating the population health impact of a multi-cancer early detection genomic blood test to complement existing screening in the US and UK. Br J Cancer, 2021. 125(10): p. 1432-1442.
- Simon, C.E., M. Assisted by Drs. Elizabeth, and R. Mary, On Auto-Antibody Formation and Antihemolysis. J Exp Med, 1909. 11(5): p. 695-717.
- Duarte, J.S., J; Mulder, N; Blackburn, J., Protein Functional Microarrays: Design, Use and Bioinformatic Analysis in Cancer Biomarker Discovery and Quantitation, in Bioinformatics of Human Proteomics, X. Wang, Editor. 2013, Springer Science+Business Media Dordrecht. p. 39-74.
- Aziz, F. and J. Blackburn, Autoantibody-Based Diagnostic Biomarkers:Technological Approaches to Discovery and Validation, in Autoantibodies and Cytokines, W.A. Khan, Editor. 2018, IntechOpen. p. 159-188.
- de Jonge, H., et al., Anti-Cancer Auto-Antibodies: Roles, Applications and Open Issues. Cancers (Basel), 2021. 13(4).
- Da Gama Duarte, J., J.M. Peyper, and J.M. Blackburn, B cells and antibody production in melanoma. Mamm Genome, 2018. 29(11-12): p. 790-805.
- Zaenker, P. and M.R. Ziman, Serologic autoantibodies as diagnostic cancer biomarkers--a review. Cancer Epidemiol Biomarkers Prev, 2013. 22(12): p. 2161-81.
- Rose, N.R., The Concept of Immunodiagnosis, in Autoantibodies (Third Edition), Y. Shoenfeld, P.L. Meroni, and M.E. Gershwin, Editors. 2014, Elsevier. p. 3-10.
- Tan, E.M. and J. Zhang, Autoantibodies to tumor-associated antigens: reporters from the immune system. Immunol Rev, 2008. 222: p. 328-40.
- Laranja, W.W., et al., The Biopsychosocial Burden of Prostate Biopsy at the Time of Its Indication, Procedure, and Pathological Report. Prostate Cancer, 2019. 2019: p. 2653708.
- Hayes Balmadrid, M.A., et al., Anxiety prior to breast biopsy: Relationships with length of time from breast biopsy recommendation to biopsy procedure and psychosocial factors. J Health Psychol, 2017. 22(5): p. 561-571.
- Tumor Markers in Common Use. 2021 [cited 2022 10/31/2022]; Available from: https://www.cancer.gov/.
- Lubin, R., et al., Serum p53 antibodies as early markers of lung cancer. Nat Med, 1995. 1(7): p. 701-2.
- Patel, A.J., et al., A highly predictive autoantibody-based biomarker panel for prognosis in early-stage NSCLC with potential therapeutic implications. Br J Cancer, 2022. 126(2): p. 238-246.
- Kratz, J.R., et al., A prognostic assay to identify patients at high risk of mortality despite small, node-negative lung tumors. JAMA, 2012. 308(16): p. 1629-31.
- Van Norman, G.A., Overcoming the Declining Trends in Innovation and Investment in Cardiovascular Therapeutics: Beyond EROOM’s Law. JACC Basic Transl Sci, 2017. 2(5): p. 613-625.
- Schulthess, D., et al., Medicine adaptive pathways to patients (MAPPs): using regulatory innovation to defeat Eroom’s law. Chin Clin Oncol, 2014. 3(2): p. 21.
- Ringel, M.S., et al., Breaking Eroom’s Law. Nat Rev Drug Discov, 2020. 19(12): p. 833-834.
- FDA Approval Timeline of Active Immunotherapies. 2021 9/7/2022 [cited 2022 10/31/2022]; Available from: https://www.cancerresearch.org/fda-approval-timeline-of-active -immunotherapies.
- Medicines. 2020 [cited 2022 10/31/2022]; Available from: https://www.ema.europa.eu/en/medicines.
- Jenkins, R.W., D.A. Barbie, and K.T. Flaherty, Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer, 2018. 118(1): p. 9-16.
- Zhang, Y., et al., Biomarkers and risk factors for the early prediction of immune-related adverse events: a review. Hum Vaccin Immunother, 2022. 18(1): p. 2018894.
- Iwama, S., T. Kobayashi, and H. Arima, Clinical Characteristics, Management, and Potential Biomarkers of Endocrine Dysfunction Induced by Immune Checkpoint Inhibitors. Endocrinol Metab (Seoul), 2021. 36(2): p. 312-321.
- Ghosh, N., et al., Autoantibodies in Patients With Immune-Related Adverse Events From Checkpoint Inhibitors: A Systematic Literature Review. J Clin Rheumatol, 2022. 28(2): p. e498-e505.
- Da Gama Duarte, J., et al., Autoantibodies May Predict Immune-Related Toxicity: Results from a Phase I Study of Intralesional Bacillus Calmette-Guerin followed by Ipilimumab in Patients with Advanced Metastatic Melanoma. Front Immunol, 2018. 9: p. 411.
- Giannicola, R., et al., Early blood rise in auto-antibodies to nuclear and smooth muscle antigens is predictive of prolonged survival and autoimmunity in metastatic-non-small cell lung cancer patients treated with PD-1 immune-check point blockade by nivolumab. Mol Clin Oncol, 2019. 11(1): p. 81-90.
- Yu, X., B. Petritis, and J. LaBaer, Advancing translational research with next-generation protein microarrays. Proteomics, 2016. 16(8): p. 1238-50.
- Van Regenmortel, M.H., Immunoinformatics may lead to a reappraisal of the nature of B cell epitopes and of the feasibility of synthetic peptide vaccines. J Mol Recognit, 2006. 19(3): p. 183-7.
- Barlow, D.J., M.S. Edwards, and J.M. Thornton, Continuous and discontinuous protein antigenic determinants. Nature, 1986. 322(6081): p. 747-8.
- Duarte, J.G. and J.M. Blackburn, Advances in the development of human protein microarrays. Expert Rev Proteomics, 2017. 14(7): p. 627-641.
- Robinson, W.H., et al., Autoantigen microarrays for multiplex characterization of autoantibody responses. Nat Med, 2002. 8(3): p. 295-301.
- Morton, D.L., et al., BCG immunotherapy of malignant melanoma: summary of a seven-year experience. Ann Surg, 1974. 180(4): p. 635-43.
- Les, I., et al., Association of immune-related adverse events induced by nivolumab with a battery of autoantibodies. Ann Med, 2021. 53(1): p. 762-769.
- Barth, D.A., et al., Evaluation of autoantibodies as predictors of treatment response and immune-related adverse events during the treatment with immune checkpoint inhibitors: A prospective longitudinal pan-cancer study. Cancer Med, 2022. 11(16): p. 3074-3083.
- Adhikari, S., et al., A high-stringency blueprint of the human proteome. Nat Commun, 2020. 11(1): p. 5301
- Yang, J., et al., Early screening and diagnosis strategies of pancreatic cancer: a comprehensive review. Cancer Commun (Lond), 2021. 41(12): p. 1257-1274.
- Peyvandi, F., et al., Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura. N Engl J Med, 2016. 374(6): p. 511-22.
- Schattner, A., The origin of autoantibodies. Immunol Lett, 1987. 14(2): p. 143-53.
- Guilbert, B., G. Dighiero, and S. Avrameas, Naturally occurring antibodies against nine common antigens in human sera. I. Detection, isolation and characterization. J Immunol, 1982. 128(6): p. 2779-87.
- Schwartz, R.S. and B.D. Stollar, Origins of anti-DNA autoantibodies. J Clin Invest, 1985. 75(2): p. 321-7.
- Klunk, J., et al., Evolution of immune genes is associated with the Black Death. Nature, 2022.
- Shah, A.A., et al., Protective Effect Against Cancer of Antibodies to the Large Subunits of Both RNA Polymerases I and III in Scleroderma. Arthritis Rheumatol, 2019. 71(9): p. 1571-1579.
- Korngold, L. and D. Pressman, The localization of antilymphosarcoma antibodies in the Murphy lymphosarcoma of the rat. Cancer Res, 1954. 14(2): p. 96-9.
- Graham, J.B. and R.M. Graham, Antibodies elicited by cancer in patients. Cancer, 1955. 8(2): p. 409-16.
- Makari, J.G., Recent studies in the immunology of cancer. III. Detection of cancer antibodies and auto-antibodies by an intradermal reaction, with a review of the detection in human serum of cancer antigens by the Schulz-Dale method. J Am Geriatr Soc, 1960. 8: p. 16-29.
- Rose, N.R., S. Shulman, and E. Witebsky, Studies of normal and malignant tissue antigens. Cancer Res, 1956. 16(9): p. 831-41.
- Ganesan, V., D.P. Ascherman, and J.S. Minden, Immunoproteomics technologies in the discovery of autoantigens in autoimmune diseases. Biomol Concepts, 2016. 7(2): p. 133-43.
- Irure-Ventura, J. and M. Lopez-Hoyos, The Past, Present, and Future in Antinuclear Antibodies (ANA). Diagnostics (Basel), 2022. 12(3).
About Sengenics
Sengenics is an immunoproteomics company working to improve patient outcomes through physiologically relevant, data-guided decision making. Our solutions enable the discovery and validation of autoantibody biomarker signatures for patient stratification, therapeutic response prediction and elucidation of disease mechanisms.
The company has a global footprint with multiple corporate and research sites across the world with customers and collaborators that include top pharma, biotech and ivy league academic institutions in North America, Europe, and Asia.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.