Sponsored Content by ByonoyReviewed by Maria OsipovaMar 18 2024
Automating microplate reading on liquid handling systems (LHSs) has brought about a substantial advancement in laboratory system workflows, seamlessly combining the precise LHS capabilities of liquid handlers with the analytical readout efficacy of the microplate reader.

Image Credit: Byonoy GmbH
This integrated approach, allowing for the simultaneous measurements of multiple samples, enhances throughput, allowing researchers to conduct their work more efficiently. The streamlined workflow helps avoid manual errors associated with cell seeding, data recording, and pipetting, ensuring the reliability and reproducibility of results.
This approach proves particularly valuable in cell-based assays, where speed, precision, and reproducibility are especially important. It also helps researchers generate high-quality data when examining complex mechanisms.
This study evaluates the performance of the on-deck integration of Absorbance 96 Automate with the epMotion® LHS for automating the CytoTox 96® non-radioactive cytotoxicity assay and compares it with another benchtop microplate reader.
Results demonstrate comparable reproducibility and optical density (OD) readouts between the integrated solution and its benchtop counterpart, validating the robustness and reliability of Absorbance 96 Automate for automated cell-based assays.
Automated cell-based assay workflow
HeLa cells were seeded into a 96-well microplate using the epMotion® LHS. After incubation in CO2, different concentrations of Staurosporine (a potent and cell-permeable inhibitor known for its impact on various protein kinases leading to cytotoxicity) were added to cells through an automated workflow and followed by an additional incubation at 37 °C in a CO2 incubator.1
The CytoTox 96® non-radioactive cytotoxicity assay kit (Promega) was used to assess induced cellular cytotoxicity. Utilizing the epMotion® LHS, CytoTox 96® substrate and stop buffer were added to the cells following the manufacturer's protocol.
Samples were automatically subjected to absorbance measurement at 490 nm using the Absorbance 96 Automate positioned on the deck of the epMotion® workstation.
Results
A comparative analysis was conducted with the GloMax® Discover plate reader (Promega) to assess the performance of the Absorbance 96 Automate. Staurosporine-treated cell samples were subjected to absorbance measurements at 490 nm using both instruments.
The results revealed similar OD values across the sample range (0 – 5 x 104 nM Staurosporine). A high correlation (R2 = 0.9997) between the readouts was observed for the two instruments.
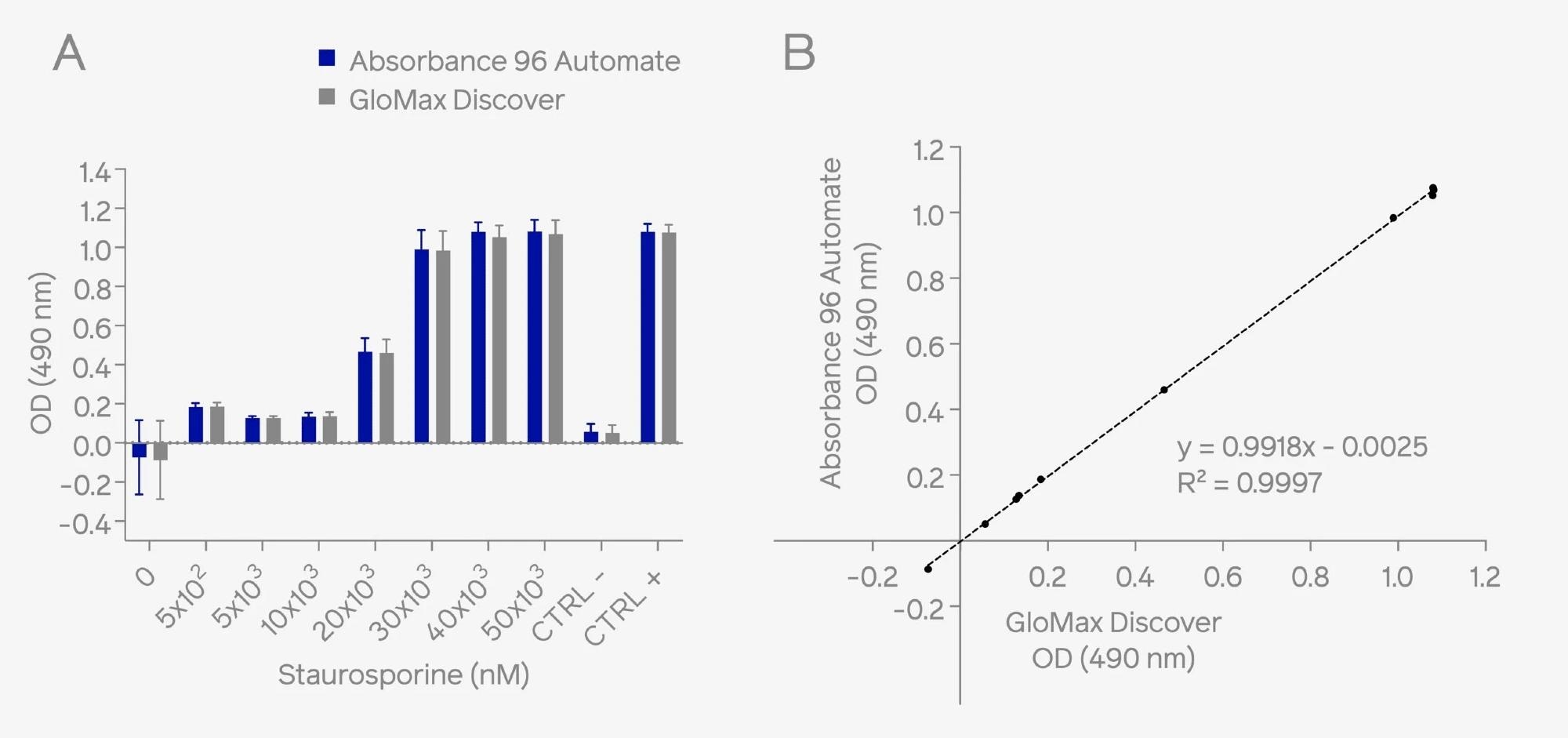
Figure 1. HeLa cells were treated with Staurosporine to induce cellular cytotoxicity. Assessment of cellular cytotoxicity was conducted using the CytoTox 96® Non-radioactive Cytotoxicity Assay kit. Absorbance measurements at 490 nm were performed using both the on-deck integrated Absorbance 96 Automate on the epMotion® liquid handling system and the benchtop microplate reader, GloMax® Discover. Results demonstrate comparable OD readouts (A) and high correlation (B) across different OD ranges. Image Credit: Byonoy GmbH
The results confirm that the Absorbance 96 Automate displays comparable sensitivity, reproducibility, and linearity within the evaluated dynamic range when compared with the GloMax® Discover plate reader.
The Absorbance 96 Automate showcased a reduced readout time compared to the GloMax® Discover (3 compared to 52 seconds), underscoring its efficiency in enabling rapid readouts.
Summary
The integration of the Absorbance 96 Automate with the epMotion® deck is characterized by its seamless nature. It offers a convenient automatic workflow for evaluating cell-based assays with precision and enabling high-throughput capabilities.
This study validates the Absorbance 96 Automate as a reliable and effective on-deck microplate reader for absorbance measurements in an automated CytoTox 96® cytotoxicity assay. It highlights its potential utility in various other cell-based assay automation workflows.
References
- Ding, Y. et al. (2017) Staurosporine suppresses survival of HepG2 cancer cells through Omi/HtrA2-mediated inhibition of PI3K/Akt signaling pathway, Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine, 39(3).
Acknowledgments
Produced from materials originally authored by Abhiyan Viplav and Rucha Datar from Eppendorf SE. Check out the original article here.
About Byonoy
The Byonoy GmbH was founded in 2015 as a spin-off from the University of Kiel and is situated in Hamburg. Our core competence is to develop innovative microplate readers based on photometric measurements for laboratory use.
Our aim is to facilitate the research and development process, to give researchers new opportunities, and to make the advantages of advanced biotechnological methods accessible to a broad range of laboratories.
For the realization of a successful innovation process, our network is a crucial factor. Our partners help us to realize our ideas and we are glad to share our expertise with them.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.