The CellTiter-Glo® 3D Cell Viability Assay is explicitly validated for assessing cell viability in 3D microtissue cultures.
- Convenient assay setup
- Precise 3D cytotoxicity assessment
- Straightforward, 30-minute procedure
The CellTiter-Glo® 3D Cell Viability Assay is tailored to assess cell viability within 3D microtissue spheroids. Its reagent effectively permeates large spheroids and possesses enhanced lytic capabilities, enabling superior viability determination compared to alternative methods.
Built upon the dependable chemistry of the original CellTiter-Glo® Assay, this innovative 3D assay reagent detects ATP as a viability indicator, producing a luminescent readout with significantly higher sensitivity than colorimetric or fluorescence-based techniques. Rapid results are achieved with a straightforward, 30-minute protocol and pre-prepared reagent.
New! Use with the New MyGlo Reagent Reader from Promega. A customized 96-well MyGlo reagent reader system incorporating instrument, assay preparation, and data processing will help users speed up and simplify cell viability tests. MyGlo detects more than just assay signals. It walks users through every step of the CellTiter-Glo® experiments.
Better ATP recovery from larger 3D microtissue spheroids
Source: Promega Corporation
| Diameter of Spheroid (μm) |
Classic CellTiter-Glo® Assay (pmol/microtissue) |
CellTiter-Glo® 3D Assay (pmol/microtissue) |
Ratio |
| 188 |
16 ± 4 |
17 ± 4 |
1.10 |
| 386 |
79 ± 3 |
94 ± 11 |
1.19 |
| 459 |
103 ± 2 |
126 ± 11 |
1.22 |
| 565 |
127 ± 3 |
178 ± 17 |
1.40 |
Better lytic capacity
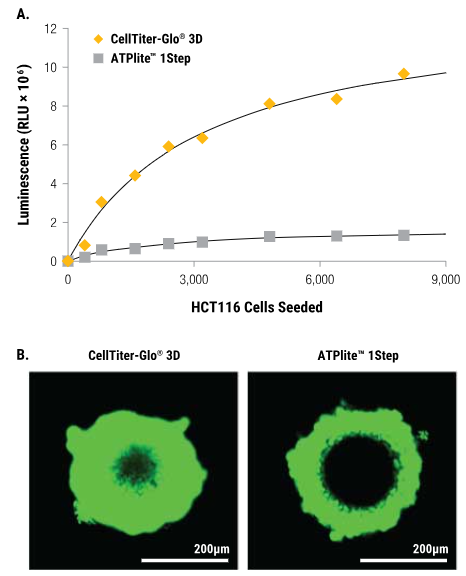
Image Credit: Promega Corporation
Improved 3D microtissue penetration, more accurate viability data
HCT116 colon cancer spheroids were created by seeding cells onto the InSphero GravityPLUS™ 96-well hanging-drop platform and cultured for four days.
Panel A
All samples received an equal volume of reagent, and after shaking for five minutes, luminescence was measured 30 minutes later.
Panel B
A 2X concentration of CellTox™ Green Dye was introduced into CellTiter-Glo® 3D Reagent (left) or ATPlite™ 1Step Reagent (right) before sample application to assess cell lysis. Images were captured at the 30-minute mark. The spheroids depicted in Panel B have a diameter of approximately 300 μm, with the bars in each image representing a distance of 200 μm.
More sensitive than colorimetric or fluorometric cell viability assays
The CellTiter-Glo® 3D Assay produces luminosity signals that are higher than the background. Some non-lytic viability assays only slightly outperform their no-cell control signals when measuring changes in fluorescence (alamarBlue®) or absorbance (MTT).
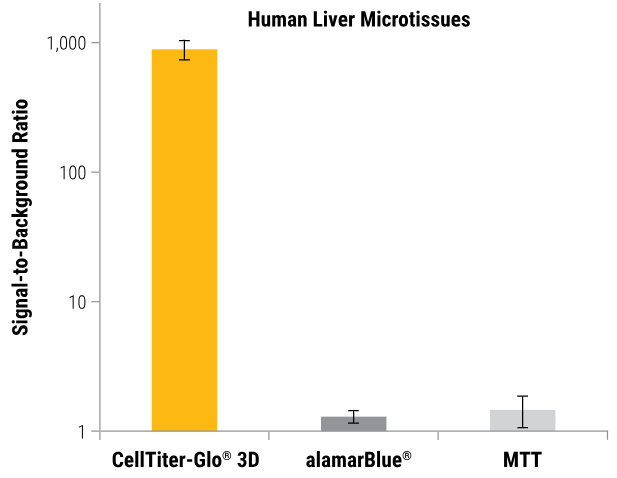
InSphero Insight™ human liver microtissues (~250μm). All microtissues were assayed using the CellTiter-Glo® 3D, alamarBlue®, and MTT assays according to the manufacturer's protocols. Total assay times for the CellTiter-Glo® 3D, alamarBlue®, and MTT assays were 30 minutes, 3 hours, and 8 hours, respectively. Image Credit: Promega Corporation
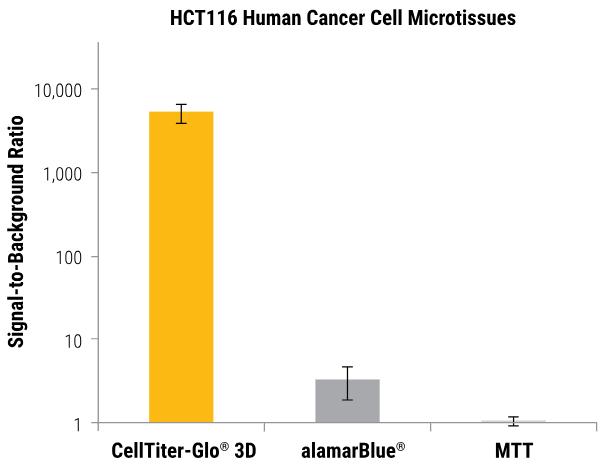
HCT116 colon cancer cells were seeded into an InSphero GravityPLUS™ 96-well hanging-drop platform and grown to generate ~340 μm spheroids. Image Credit: Promega Corporation
Compatible with a variety of 3D culture methods
The outcomes of compound screening conducted via the CellTiter-Glo® 3D Assay in hanging-drop, ultra-low attachment plate (ULA), and Matrigel® 3D cultures are depicted below. Comparable results were obtained across all three methodologies.
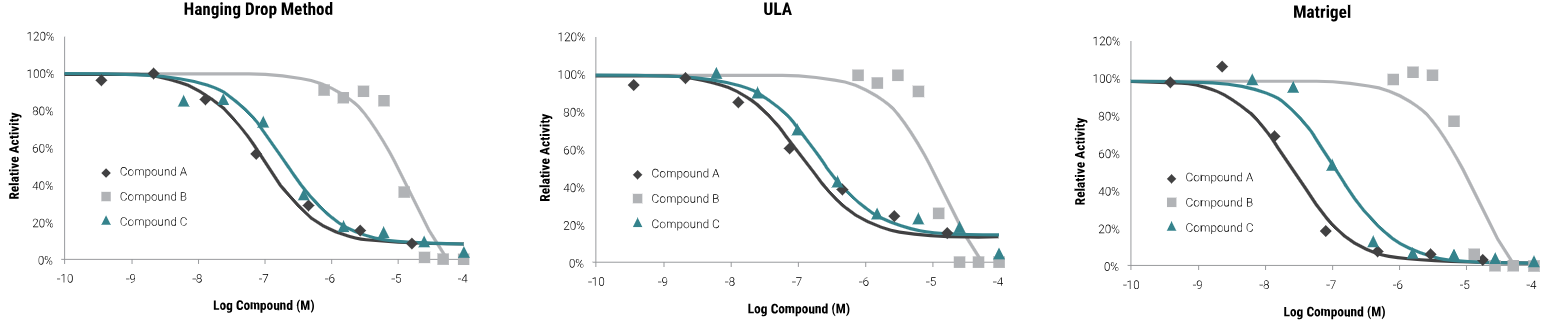
HCT116 colon cancer cells were seeded as follows: 400 cells in hanging-drop; 1,000 cells in ULA or Matrigel®. Microtissues were grown for four days, treated with compounds for 48 hours, and then assayed with the CellTiter-Glo® 3D Reagent. Luminescence was recorded at 30 minutes. Image Credit: Promega Corporation
The CellTiter-Glo® 3D Assay demonstrates excellent precision
Z'-factor experiment with 3D microtissues
Four hundred HCT116 colon cancer cells were seeded into each of the 60 wells of a 96-well InSphero GravityPLUS™ hanging-drop plate and incubated for four days to form 60 spheroids (~350 μm in diameter).
Half of the spheroids received treatment with 100 μM panobinostat (black squares), while the remaining half were treated with vehicle (1% DMSO, orange squares). After 48 hours, all samples underwent assessment with the CellTiter-Glo® 3D Reagent, yielding a Z´-factor of 0.81.
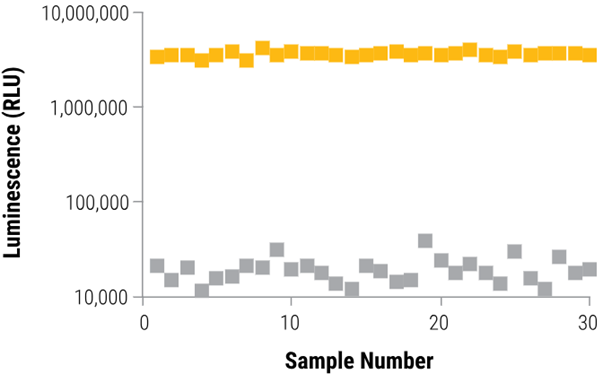
Image Credit: Promega Corporation
Specifications
What is in the box?
Source: Promega Corporation
| Item |
Part # |
Size |
| CellTiter-Glo® 3D Reagent |
G968A |
1 × 10 ml |