Get ready to explore the fascinating world of lipid nanoparticles in the latest episode of omg OMx! Kate Stumpo chats with George Mason University’s Michael Girgis as they unravel the mysteries of lipid nanoparticles (LNP). From groundbreaking research to cutting-edge applications, they'll reveal why studying these tiny particles is crucial for our future. Don't miss out on this must-see episode - read the highlights or watch it now!
Michael Girgis | omg OMx Podcast | Ep. 3
Can you tell us in simpler terms what LNPs are and why they are so groundbreaking in medicine?
Lipid nanoparticles are like tiny superheroes in medicine, solving many problems that have challenged drug delivery for years.
These nanoparticles are made up of several lipids, including an essential ionizable lipid, as well as helper lipids like DSPC and cholesterol, and PEGylated lipids. These components are mixed in ethanol with the cargo, which can be RNA or other types of drugs.
Creating these particles is a complex process involving microfluidics fusing. It all starts with the ionizable lipids, which are positively charged and associate with the negatively charged RNA or DNA. Then, the helper lipids form the first layer, followed by more layers to create the lipid bilayer. Finally, PEGylated or polyethylene micro-lipids partition themselves to ensure the hydrophobic and hydrophilic sections are outside, which helps with dissolution.
Lipid nanoparticles have been a game-changer for delivering RNA and peptide-based drugs that were once destroyed by nucleolus or RNAs and DNAs in the plasma. These tiny particles can safely transport medication to the cell or through the cellular membrane to the cytoplasm, overcoming many of the barriers previously faced.
In fact, these nanoparticles have numerous applications in delivering RNA and DNA-based drugs, peptides, and proteins. At around 60 to 80 nanometers in size, they can efficiently deliver drugs to various parts and through the lipid bilayer of the cellular membrane, making them a vital tool in drug delivery systems.
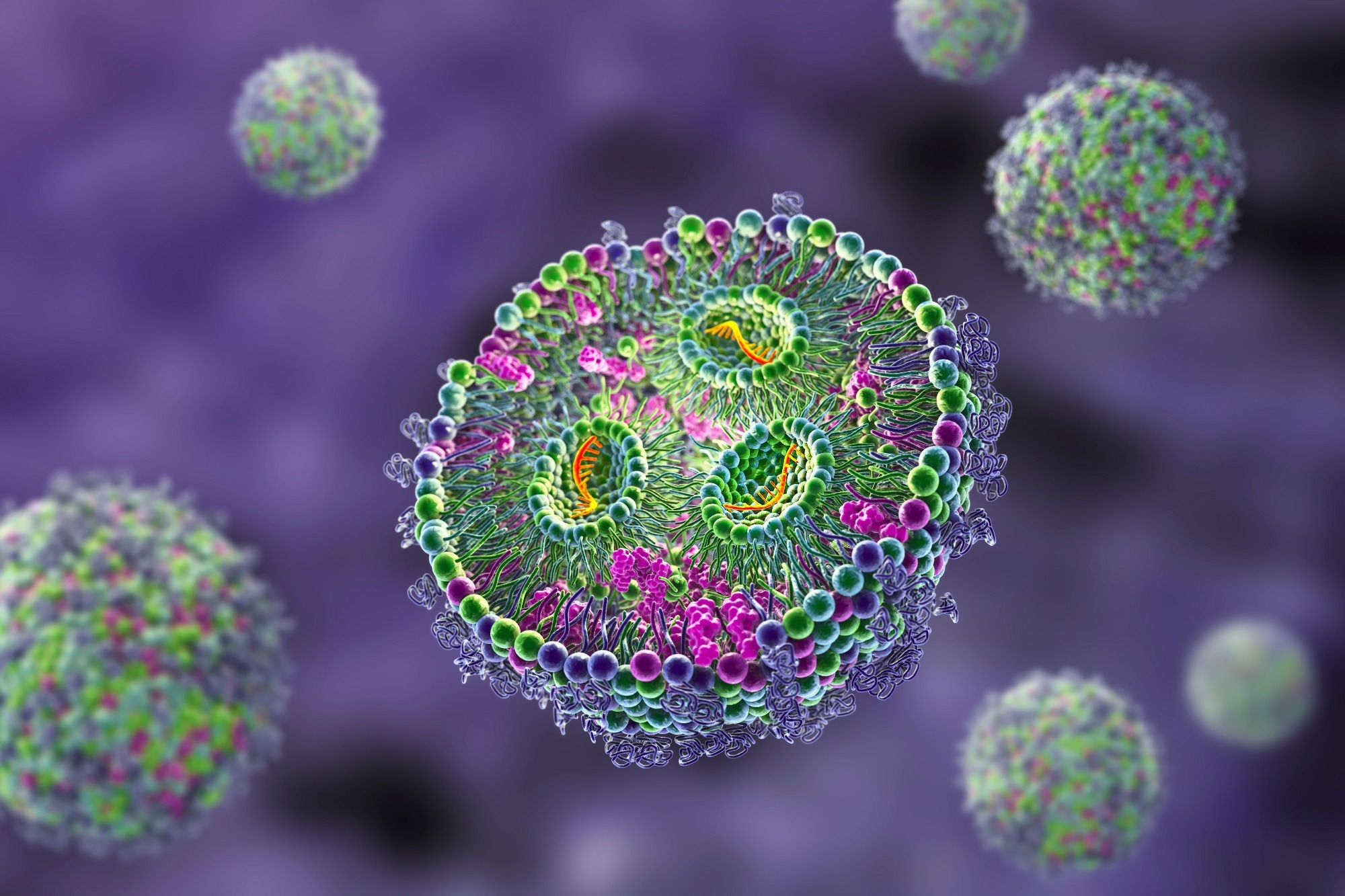 Image credit: Shutterstock / Kateryna Kon
Image credit: Shutterstock / Kateryna Kon
How are lipid nanoparticles being used in real-world applications and what's the status of FDA approval for these drugs?
Lipid nanoparticles have truly made a remarkable impact on medicine, especially with the development of mRNA vaccines by companies like Moderna and Pfizer, which have been working on this for over 20 years. These nanoparticles encapsulate mRNA and deliver them to cells via endocytosis, where proteins are generated, triggering an immune response that helps build immunity.
The success of these vaccines is nothing short of incredible, but it's not just COVID-19 that lipid nanoparticles are making a difference with. For example, Onpattro is a drug that uses short-interfering RNA inside lipid nanoparticles to treat a rare genetic disorder. This is just one example of how lipid nanoparticles are being used to control gene expression, revolutionizing the way we approach certain diseases.
Lipid nanoparticles can also deliver drugs that are difficult to solubilize, like anti-cancer drugs, such as Iclusig, and even smaller molecules like doxycycline. When formulated into lipid nanoparticles, doxycycline can be used to treat certain types of cancer.
Furthermore, Onivyde is a widely-used drug in solid tumors to deliver anti-cancer medications to tumors effectively. The applications for using lipid nanoparticles in drug delivery are numerous, and we are thrilled to be part of this medical revolution.
Can you tell us about a moment in your career that was particularly exciting or rewarding for you, and how did it shape and fuel your curiosity?
The moment that changed me personally was when I contributed to identifying an unknown biomarker resulting from radiation exposure. It was a mysterious and challenging task as we could not find a match for this biomarker based on the mass spec or other sources of information.
However, after working for two to three months, I proposed a structure based on a fragmentation pattern. I presented my findings to my supervisor and suggested we invest in synthesizing the molecule to prove my theory. Eventually, we got it synthesized, and when I saw the same fragmentation and retention times, I knew I had identified a brand-new biomarker.
It was an incredible feeling, highlighting how mass spectrometry is crucial in identifying and characterizing various biological molecules, allowing us to understand biological pathways and entities. Knowledge is power. It's amazing how knowledge can drive progress, and I believe that mass spectrometry is a crucial tool in advancing our understanding of biology.
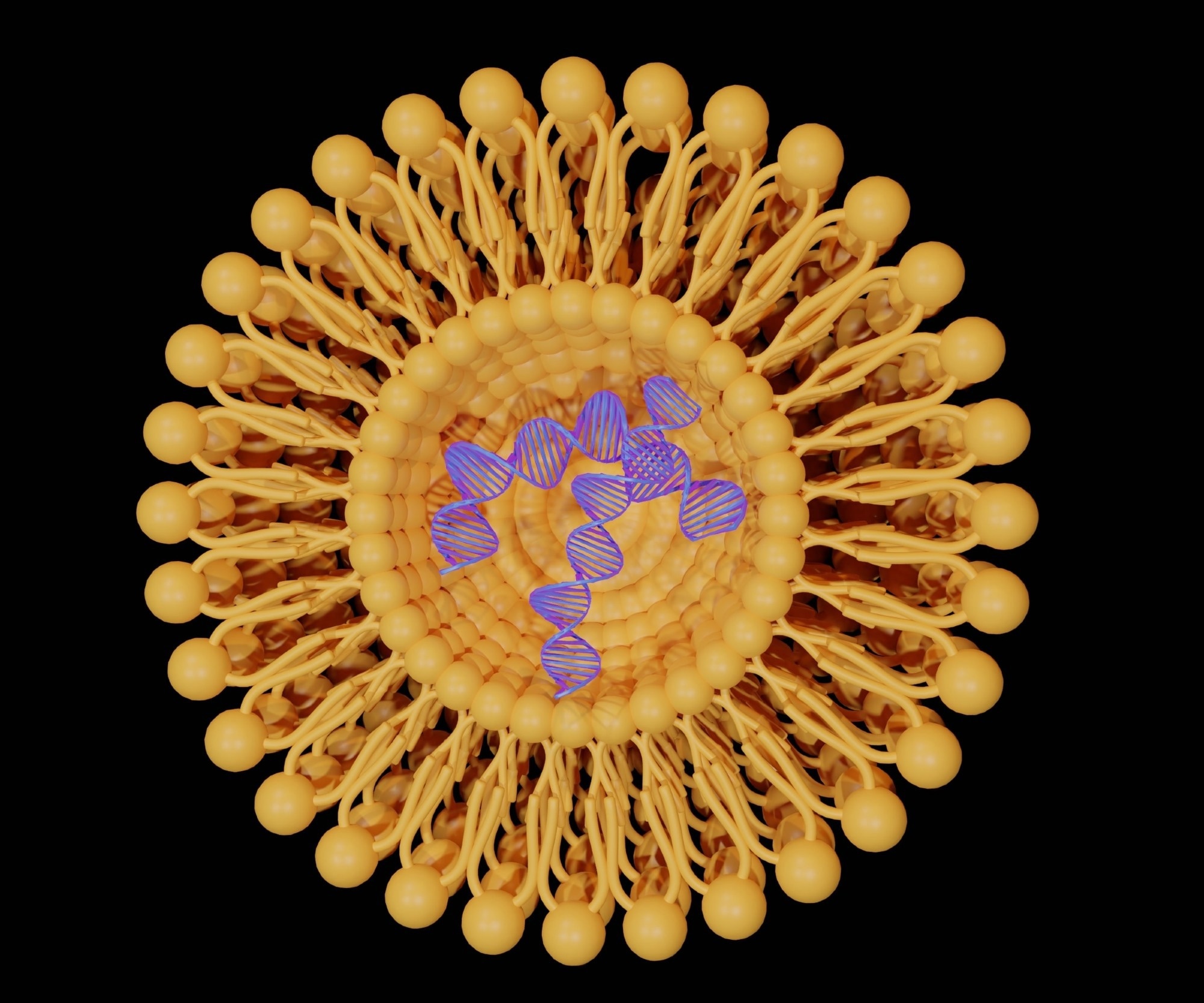 Image credit: Shutterstock / Love Employee
Image credit: Shutterstock / Love Employee
Can you explain to us why mass spectrometry is critical in this field, and what is your preferred method for characterizing LNPs?
In the process of self-assembling LNPs, there are many potential points of failure. For example, if the buffer is inappropriate, or RNA is degraded or contains impurities, the LNP formulation may be compromised. This is where mass spectrometry plays a crucial role. By characterizing the starting material, we can identify any impurities present and understand their concentration, as the FDA has strict rules for characterizing impurities.
Lipid degradation is a particular concern, and it is crucial to know where oxidation occurs, whether it's on the head, tail end, or connecting portion of the lipid. Understanding this information is essential in determining whether the study materials qualify for use in LNP formulation. Additionally, stability and storage conditions are also vital factors in the formulation process, particularly for developing countries without refrigeration facilities.
To answer all of these questions, we use force degradation studies to screen for potential problems with the LNP and RNA. Various instrumentation is required to obtain the necessary information. Overall, mass spectrometry is a powerful tool that helps researchers understand the biological pathways and entities involved in the LNP self-assembly process, ultimately advancing our understanding of biology.
About the speaker

Michael Girgis, Assistant Professor at George Mason University's Bioengineering department
Michael is a synthetic organic chemist & an experienced spectroscopist Ph.D. candidate with extensive hands-on experience conducting research at the GMU Biochemistry and Chemistry department.

omg OMx host: Kate Stumpo, Senior Market Manager at Bruker
LinkedIn
About Bruker Life Sciences Mass Spectrometry

Discover new ways to apply mass spectrometry to today’s most pressing analytical challenges. Innovations such as Trapped Ion Mobility (TIMS), smartbeam and scanning lasers for MALDI-MS Imaging that deliver true pixel fidelity, and eXtreme Resolution FTMS (XR) technology capable to reveal Isotopic Fine Structure (IFS) signatures are pushing scientific exploration to new heights. Bruker's mass spectrometry solutions enable scientists to make breakthrough discoveries and gain deeper insights.