Introduction
Luminescence reagents and assays are often used in drug discovery and research, but over the last decade their use has increased considerably owing to the high specificity of assays, ease of use, and good sensitivity at low levels of screened compounds.
Contemporary photometric instruments can precisely determine very low levels of photon emissions from luminescence substrates, which has increasingly focused the spotlight on the optical cross-talk inherent in the SBS/ANSI standard microplate design and the signal-to-noise ratio that can be achieved.
This article compares the optical cross-talk that can be observed in micro well plates produced by three different manufacturers and demonstrates that a black supporting matrix can be used to enhance sensitivity in microplate assays.
Photometric Methods
First, the study compares two solid white polystyrene shallow well microplates from two different manufacturers with the Porvair Sciences’ patented design which combines white individual wells with a solid black plate matrix, as shown in Figure 1.

Figure 1. The unique Porvair black & white 96-well assay plate
For comparison, a luminescent assay using Firefly luciferase was utilized. Three fundamental techniques are available for achieving valuable optical information from microplate-based samples. The simplest technique is absorbance measurement. Based on the Beer-Lambert law, the concentration of a given compound in solution is directly proportional to the quanta of light absorbed at a constant optical path length and at a given wavelength. Therefore, it is possible to direct a monochromatic light beam into the well and determine the absorbed or transmitted light. The relative concentration in each well can then be estimated using a simple calculation. This technique is used by most laboratory microplate readers.
Fluorescence measurements are performed when greater sensitivity is needed. In this experiment, an excitation beam of a given wavelength is directed into the well. Photons from the incident light are absorbed by the fluorophore, which reproduces photons of lower energy that then appear at a longer wavelength. In a fluorimeter, cut-off filters can be used to eliminate excitation wavelengths from the detected light, meaning only the emission wavelength is left. When compared to simple absorbance measurements, the sensitivity achieved can be up to ten times greater. In order to achieve greater sensitivity, the band-width of the emission and excitation wavelengths can be reduced by using monochromators.
Bioluminescence
Some plant and animal species can produce light via one of two mechanisms – the luciferin and aequorin pathways. This natural phenomenon is referred to as bioluminescence. Certain species such as the Sea Pansy, firefly beetles, and the marine copepod Metrida Longa all have luciferase enzymes, which catalyze oxidation of luciferin substrate in the presence of adenosine triphosphate (ATP) and result in light being emitted.
When cells are transfected with a generic construct containing a luciferase gene, luciferase can be used to evaluate transcription activity inside cells or to provide a highly sensitive assay for cellular ATP levels utilized to determine cell apoptosis or cell death in high throughput screening (HTS) applications. In such assays, the cloned luciferase enzyme system produces a continuous glow and the kinetics of this can be analyzed and linked to, for example, the concentration of ATP or a cell’s propensity to divide, remain stable or undergo cell apoptosis in response to chemical stimuli. A number of leading Life Science companies market these types of luminescence assays, some of which include OneGloTM, ATPLiteTM, LucLiteTM, and AequoScreenTM. Detection is usually achieved using a luminometer or a multi-label reader, which is a multi-purpose spectrometer that can read luminescence, fluorescence or absorbance modes. Figure 2 shows a schematic diagram of the three optical techniques of detection.

Figure 2: Photometric detection methods
Microplate Design Considerations
The microplates used in HTS and assay development are usually made from polystyrene polymer. Titanium dioxide (TiO2) serves as an optical brightener which is added to this polymer to enhance the surface reflectivity of white plates. The tools used to mould these plates are also well polished to ensure a very bright, smooth surface within the wells. These measures are taken to ensure that the generated light inside the wells is reflected towards the top of the micro well. This is where the highly sensitive optical detector of the measuring instrument is positioned at the time of the reading cycle. Ideally, a photodiode detector or photomultiplier would capture most of the light generated and reflected in this way. In the case of fluorescence measurements, it is essential to quench the auto-fluorescence emitted by the polystyrene substrate and this can be achieved by adding 1% carbon black to the polystyrene. Such an approach has proved highly effective in fluorescence assays and provides better results when compared to a white plate. In order to improve reflectivity, all the interior surfaces are again also polished.
However, difficulties may arise when luciferase assays are used for screening, where a high dynamic range is observed across the plate. In these cases, very weak or strong signals may be exhibited by adjacent wells, which can result in optical cross-talk between them and therefore incorrect results. A certain amount of visible light may get through the plate’s plastic walls and be incorrectly detected in adjacent cells as an extra signal. The error is most pronounced when reading a low level signal next to a much brighter well. Plate designs that use isolated chimney wells can help to minimize this, but they cannot eliminate the problem completely.
In order to address this issue of light-piping by the white plastic, Porvair Sciences developed the 96-well Black and White plate. During production, individual white cells are moulded into a black polystyrene plate matrix, in what is referred to as a two shot process. This process is also used to mould clear wells into one of a black or white matrix to produce the Krystal 2000TM Zero-cross-talk plates supplied by Porvair Sciences for bottom-reading fluorescence and absorbance purposes.
When bright white wells comprising up to 18% TiO2 are integrated into a 1% carbon black matrix, optical crosstalk is all but eliminated in the new plate. In contrast to solid white assay plates from two other manufacturers, Porvair’s black and white plate has 82% and 56% lower average well-to-well cross-talk, respectively.
Research Methodology
BMG Labtech’s POLARStar Omega multi-mode spectrophotometer was used to take readings at 510nm wavelength using 5µl of a solution containing Firefly luciferase and luciferin introduced into the C4 well of each 96-well plate. To start the reaction, a 5µL aliquot of hydrogen peroxide was then added. To allow for the luminescent signal decaying over time, four readings, each lasting 1 second, were obtained from each of the surrounding, empty wells and then averaged.
These are illustrated in the data as Cycle 1, 2, 3, and 4, with each representing a single minute of the time elapsed since the initial reading was obtained. This is important because the luciferase signal decays rapidly in 8 to 10 minutes and readings are therefore obtained until the signal has fallen to nearly 50% of initial levels.
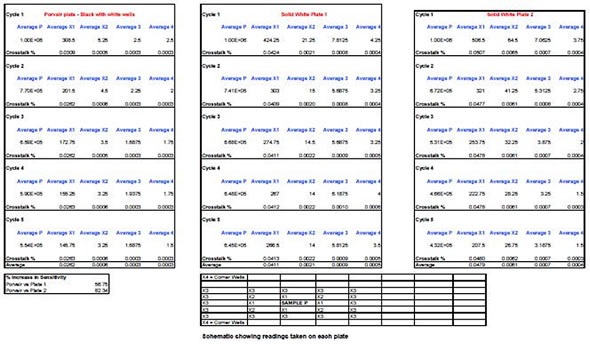
Figure 3. Schematic showing the position of the sample and the various measurement positions around the sample.
For each individual experiment, one plate was prepared and only a fresh, clean plate was used from a sealed pack. This procedure was again carried out for the other manufacturers’ plates. In order to obtain a background reading for the signal-to-noise, the same type of empty plate was used.
Results
The signal-to-noise ratio calculated for the 96-well black and white plate was 192000:1, compared with 178000:1 for a white plate. This demonstrates a useful 7.5% increase in detectable signal over the background. For wells closest to the reaction mixture (Cells X1), the calculated cross-talk was just 0.026% in the black and white plate, compared with 0.048% and 0.041% in the other two solid white plates, which translates as an 82% improvement.
In luminescence crosstalk experiments, it is not useful to run a blank with only water or buffer, because no visible signal will be observed. If such an experiment was performed, readings taken from the photo-detector would be either the result of dark current or stray light penetrating the sample compartment. Dark current refers to the constant quantum noise from the elements and electronics of the detector that is automatically corrected by the instrument firmware.
Conclusion
The above results demonstrate that the composite black and white plate from Porvair Sciences is very effective for determining low-level luciferase-based assays in drug development and screening applications. The combination of increased reflectivity from TiO2 brightener and successful quenching by carbon black provides a better intra-plate dynamic range and an improved signal-to-noise ratio. This allows screeners to screen for weaker hits, with reduced concentrations of reagents or at lower detection levels.
Acknowledgement
Produced from articles authored by Stephen Knight BSc. MA, Director of Sales & Marketing, Porvair Sciences Ltd, Wrexham, United Kingdom.
About Porvair Sciences
Porvair Sciences, specialists in the manufacture of microplate products, serve Life Sciences, Biotechnology, R&D and Molecular Biology with microplate solutions for all applications, from sample preparation to high throughput screening via our global distributor network.
Our range includes vacuum manifolds, sealers, evaporators and microtiter plates in all popular styles; deep well and shallow well storage plates, assay plates, luciferase reporter gene plates and liquid handling reagent reservoirs. We also provide custom microplate products for life science research. Our vacuum manifolds, essential for 96-well SPE sample preparation, are designed to work with most popular filter plates, including Waters, Millipore, Qiagen, Whatman, GE Healthcare, Varian, Biotage and, of course, Porvair.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.