The genetic sub-classification of primary brain tumors (e.g. gliomas), is crucial for clinical decision-making, prognostication, and pathological diagnosis. Immunohistochemistry, and other genetic classification methods currently being used can take a number of days to complete.
Easy to use hardware and repeatable protocols are required to assist adoption of Raman spectroscopy into a clinical environment. Numerous publications have shown that it can give accurate diagnostics within minutes, for a range of cancer and non-cancer diseases.
Case Study
Using the Renishaw Biological Analyzer - RA816 is it possible to demonstrate differences between healthy and diseased brain tissue. This was done in a study conducted with the Neuropathology Department at the University of Oxford in England.
Medical research using Raman Spectroscopy
In this study it was possible to:
- Identify healthy and diseased tissues with no need for disease marker targeting and discovery
- Differentiate between tissue types by their overall chemical signatures – colorimetric, fluorescent or labeling were not needed
This study demonstrated how the system could support pathology decision making by:
- Using grooved mirror stainless steel slides for optimized sample measurements with brain tissue samples
- Building and testing glioma classification models (from fresh, frozen and fixed tissue, as well as brain cancer cell lines), in clinical environments, for research purposes
- Generating high-resolution images from unstained sections that can be correlated with clinical H&E and IHC stained slide images
- Discriminating the glioma cancer subtypes, in a number of tissue and cell types, with high sensitivity and specificity
- The potential to aid in defining tumor margins
- Identifying biochemical changes associated with cancer formation and progression
The Renishaw Biological Analyzer - RA816
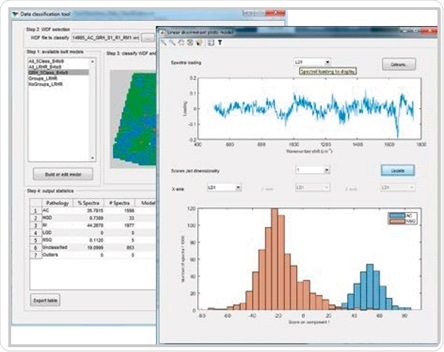
Renishaw Data Classification application developed for building, testing and validating cancer classification models
Results
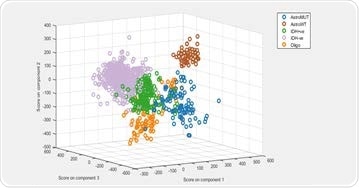
Principal component-linear discriminant analysis (PCA-LDA) was used to build a supervised classification model. The model demonstrated 80% to 95% sensitivity and specificity for predicting the five glioma genetic subtypes.
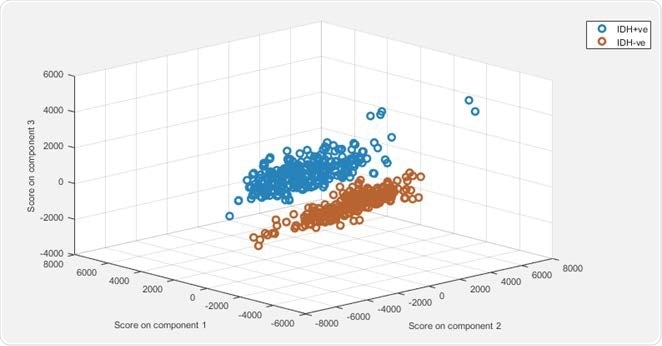
Principle component-linear discriminant analysis (PCA-LDA) modelling demonstrated 100% sensitivity and 99% specificity for predicting the presence of IDH mutations.
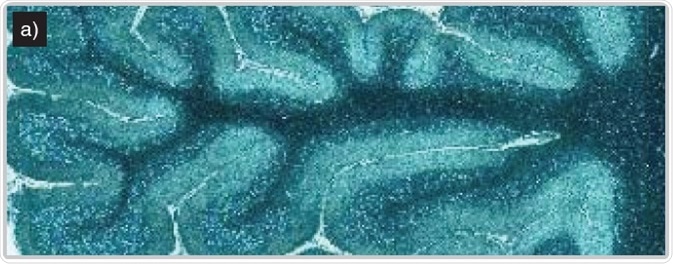
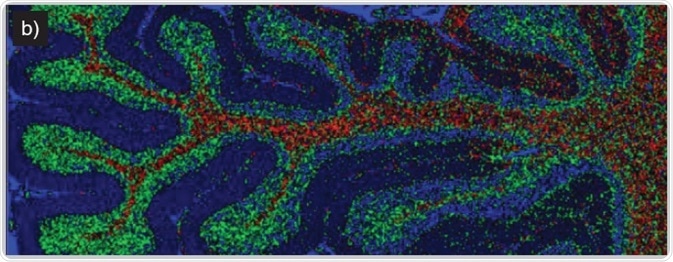
Imaging of human brain tissue. Comparison of (a) white light and (b) Raman-false-coloured composite score image of cerebellum whole follicle showing arbor vitae/white matter (red), granule cell layer (green), molecular layer (dark blue) and meninges (pia, arachnoid and dura mater) (cyan)
The ideal tissue imaging tool to meet the particular challenges of the clinical research environment; the Renishaw biological analyzer
- Supports data and model transferability for disease and pathology classification on different systems
- StreamLine™ technology permits high speed mapping with no sample damage
- Simple to use accessories and hardware targeted for bio-samples
- Optimal light microscopy performance for both macro and high magnification
- Transportable and compact
- Easy to use software that makes measurements accessible to all users without compromising performance
- Optimized hardware for stable, repeatable, and reliable, high quality data acquisition
The Renishaw Biological Analyzer is designed for Research Use Only (RUO) and not for use in diagnostic procedures.
Acknowledgments
Renishaw thanks Mr James Livermore, Oxford Radcliffe Hospital for providing the data.
References
Livermore J, Isabelle M et al, 2018. Genetic classification of gliomas using Raman spectroscopy. Poster. Cancer Research UK Brain Tumour Conference 2018
Livermore J, Isabelle M et al, 2018. Manuscript in preparation
About Renishaw

Renishaw is a global company and a recognised leader in Raman spectroscopy. Offering high performance optical spectroscopy products for over 20 years, Renishaw’s Spectroscopy Division is passionate about manufacturing the very best Raman products. It has a team of scientists and engineers specialising in the development, application and production of high performance, configurable Raman spectrometers.
With a foundation built on sound engineering, these systems offer the highest levels of flexibility and performance and are used by academics and industrialists to tackle analytical problems across a broad range of application areas, including life science, chemicals; materials science; pharmaceuticals; semiconductors; forensics; gemmology; antiquities; and green energy, such as photovoltaics.
Renishaw’s Raman systems are easy to use and produce repeatable, reliable data, even from challenging samples. We aim to be a long-term partner, offering quality products that meet customers’ needs, both today and into the future, backed up by expert technical and commercial support.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.