Identifying the existence and level of penetration of drug compounds in skin can be difficult. Adequate penetration through layers of dermal tissue cannot be accomplished using current technologies. Using the Renishaw Biological Analyzer - RA816, Raman spectroscopy established the presence of a topical compound in the epidermis and reticular dermis with high specificity and sensitivity.
In this study, Raman spectroscopy also explained the changes in the secondary structure conformation of protein peaks (ɑ-helix to β-sheet) due to biochemical changes taking place during tissue remodeling following treatment.
Case Study
The system showed the existence and penetration depth of a compound in different skin tissue layers following application of a topical formulation. This was proven in a study conducted with Miss Rubinder Basson from the University of Manchester, Division of Musculoskeletal and Dermatological Sciences.
This research comprised of a blind, randomized clinical trial of 45 people receiving an active topical (MEBO scar topical wound healing cream) against control with sequential punch biopsies. Afterwards, they were assessed using:
- The Renishaw Biological Analyzer
- qPCR gene expression
- Standard skin histology
- Optical coherence tomography
- Spectrophotometric intracutaneous analysis
- Full-field laser perfusion imaging (elasticity and hydration)
Medical research using Raman Spectroscopy
Subjects were arranged into four groups, each of which represented a point in time.
On ‘Day 0’, a 5 mm punch biopsy was performed under local anesthetic from each upper inner arm to produce a scar. The first biopsy measurements of the scar were obtained (spectroscopic imaging, histology and qPCR) at ‘Day 14’, and the people were given both control and active topicals. Measurements on the four groups were carried out after daily use of the topical, at time points of 4, 8, 12 and 16 weeks respectively.
Biopsies were either snap frozen and stored at -80 °C so that they could be used for Raman spectroscopy at a later date, or bisected and stored in formalin for RNA analysis. Snap frozen samples were cut using a cryostat at 10 μm thickness, in cross-sectional slices and mounted on calcium fluoride slides for analysis using the Renishaw Biological Analyzer.
The Renishaw Biological Analyzer - RA816
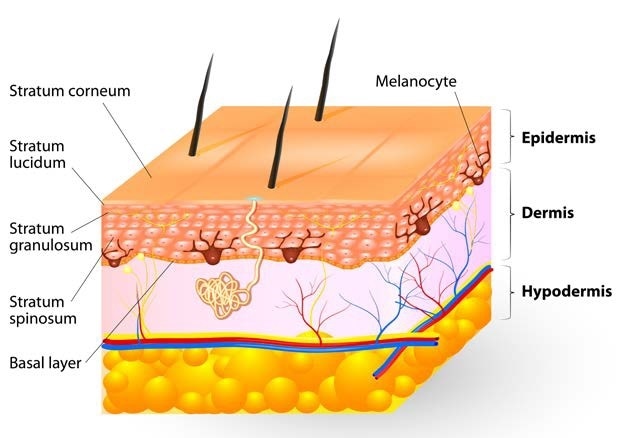
Tissue layers of the human skin
Results: Transdermal Delivery
Transdermal delivery of the active topical was seen in the epidermis and deep reticular dermis, and over sequential time points weeks 4-16, (98% and 99% specificity, 89% and 93% sensitivity, 96%, and 97% accuracy, respectively) within the scar biopsies. The existence of the topical drug compound within ‘normal skin’ spectra was confirmed using Raman spectroscopy.
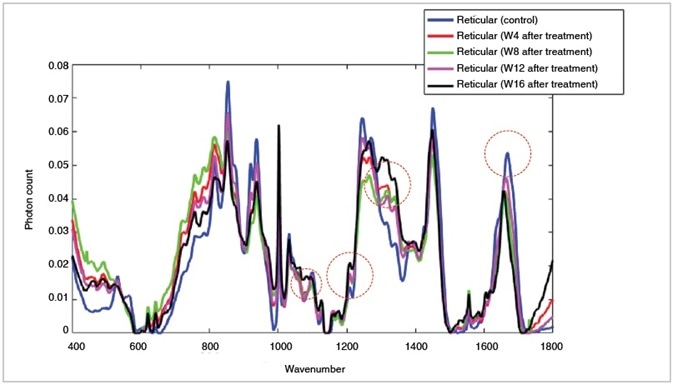
Meboscar™ compound was detected at higher level accumulation in the reticular dermis over sequential time points following treatment (day 0 to weeks 8-16) shown on the above plot at 1070 cm-1, 1210 cm-1, 1303 cm-1 and 1656 cm-1
Results: Tissue Remodeling
The levels of laminin, collagen I and III, and fibronectin, all increase as expected in raised dermal scar tissue throughout the wound healing process. Raman spectroscopy revealed a shift of the amide I peak from 1667 cm-1 (β-sheet contribution of elastin) to 1656 cm-1 (triple helix contribution of collagen types I and III) in the papillary and reticular layers of the dermis, emphasizing the changes happening throughout tissue remodeling, after treatment at week 16 in the active group.
Non-invasive calculations of elasticity obtained with a full-field laser perfusion imager were consistent with these Raman results, demonstrating a reduction in elasticity measurements in week 16.
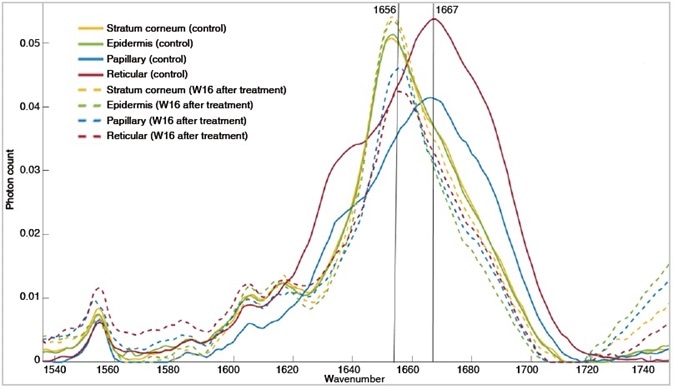
Amide I spectral region showing conformational change in protein structure from β-sheet contribution of elastin to the a-triple helix component of collagen. Observing effects of these compounds in wound-healing and tissue remodeling.
Results
The Renishaw Biological Analyzer could:
- Provide histological images without labeling
- Confirm the diffusion of the topical active ingredient into different dermal layers
- Distinguish tissue layers by their overall chemical signatures – fluorescent or colorimetric labeling was not required
- Indicate a change in skin protein composition during the healing process
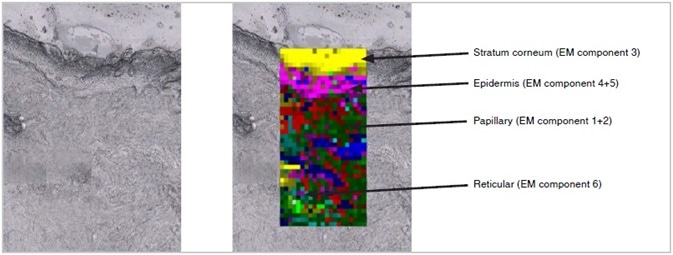
Tiled image of the unstained tissue section (left) and corresponding Empty modeling ™ processed Raman image (right) overlaid on the unstained tissue section clearly showing the different tissue layers in the skin
The Renishaw Biological Analyzer; The Ideal Raman Tissue Imaging Tool to Meet the Specific Challenges of the Clinical Research Environment
- Compact and transportable
- Optimized hardware for stable, repeatable, and high signal to noise spectral data acquisition
- Easy to use hardware operation and accessories targeted for bio-samples
- StreamLine™ technology allows high speed mapping without causing sample damage
- Optimal light microscopy performance for macro and high magnification
- Supports model and data transferability for disease and pathology classification on different systems
- Easy to use software that makes measurements accessible to all users without compromising performance
The Renishaw Biological Analyzer is designed for Research Use Only (RUO) and not for use in diagnostic procedures.
Acknowledgments
Renishaw thanks Miss Rubinder Basson, Plastic & Reconstructive Surgery Research (PRSR), Faculty of Biology, Medicine & Health, University of Manchester, UK for providing the tissue.
References
Basson R, Isabelle M et al. In vivo functional testing of a topical using a three-tiered approach in human skin enables objective and quantitative evaluation of its role in skin scarring. Manuscript in preparation
About Renishaw

Renishaw is a global company and a recognised leader in Raman spectroscopy. Offering high performance optical spectroscopy products for over 20 years, Renishaw’s Spectroscopy Division is passionate about manufacturing the very best Raman products. It has a team of scientists and engineers specialising in the development, application and production of high performance, configurable Raman spectrometers.
With a foundation built on sound engineering, these systems offer the highest levels of flexibility and performance and are used by academics and industrialists to tackle analytical problems across a broad range of application areas, including life science, chemicals; materials science; pharmaceuticals; semiconductors; forensics; gemmology; antiquities; and green energy, such as photovoltaics.
Renishaw’s Raman systems are easy to use and produce repeatable, reliable data, even from challenging samples. We aim to be a long-term partner, offering quality products that meet customers’ needs, both today and into the future, backed up by expert technical and commercial support.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.