The trimeric spike protein (S) present in SARS-CoV-2 plays a crucial part in the early stages of COVID-19 infection. The S1 domain is accountable for receptor binding and the S2 domain mediates membrane fusion.1-3
Activated by the receptor binding, the spike protein experiences a transformation from a metastable prefusion state to a stable post-fusion state throughout the infection phase.
During this phase, the receptor-binding subunit is cleaved by the protease like TMPRSS2, and the fusion subunit is subjected to large-scale conformational realignments to reveal the hydrophobic fusion peptide.4,5 Therefore, the receptor-binding domain (RBD) has become a primary target for developing therapeutic treatments for COVID-19.
Moreover, there is an additional part of the S1 protein known as the N terminal domain (NTD). While the role of NTD is not well marked, in some coronaviruses, the NTD may identify specific sugar moieties upon early attachment and have a contribution during the infection.6-8 Therefore, RBD should not be addressed as the only target, as the NTD could become a possible target for treating SARS-CoV-2.9
In recent times, a team from China led by Dr. Chen supported this idea having released their work in the publication Science. They targeted and separated human mAbs from 10 Chinese patients that had recovered from a previous COVID-19 infection. Throughout their study, there was a mAb with a great affinity to SARS-CoV-2 RBD but failed in its opportunity to effectively neutralize authentic SARS-CoV-2.
Additionally, there is an alternative NTD binding mAb (4A8) demonstrating an increased level of neutralization against SARS-CoV-2 in vitro but fails to block the interaction between ACE2 and S protein (Figure 1). This study implies the presence of other mechanisms vital for SARS-CoV-2 neutralization in addition to inhibiting the viral interaction with the receptor.
Researchers and scientists should contemplate the entire Spike protein rather than just RBD when preparing therapeutics for development. Through a combination of the NTD targeting antibodies and RBD targeting antibodies, a promising cocktail therapy may evade the mutations of viruses that manage to escape.10
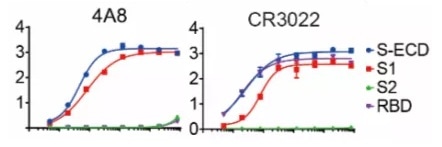
Figure 1. Binding curves of representative mAbs.
ACROBiosystems developed SARS-CoV-2 S1 NTD protein (Cat. No. S1D-C52H6), which can bind particularly well to anti-SARS-CoV-2 S1 NTD antibody (Cat. No. SPD-M121). The bioactivity of the S1 NTD protein has been verified through ELISA, BLI, and SPR assays. They are ideal for immunization, screening, and determination of therapeutic development.
References and Further Reading
- Gallagher TM, Buchmeier MJ, Coronavirus spike proteins in viral entry and pathogenesis, Virology, 2001 Jan 20; 279(2):371-4.
- Simmons G, et al., Proteolytic activation of the SARS-coronavirus spike protein: cutting enzymes at the cutting edge of antiviral research, Antiviral Res. 2013 Dec; 100(3):605-14.
- Belouzard S, et al., Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites, Proc Natl Acad Sci USA 2009 Apr 7; 106(14):5871-6.
- Song W, et al., Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2, PLoS Pathog. 2018 Aug; 14(8): e1007236.
- Hoffmann M, et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell, 2020 Apr 16; 181(2):271-280.e8.
- Wrapp D, et al., Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation, Science, 2020 Mar 13; 367(6483):1260-1263.
- Walls AC, et al., Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein, Cell, 2020 Apr 16; 181(2):281-292.e6.
- Krempl C, et al., Point mutations in the S protein connect the sialic acid binding activity with the enteropathogenicity of transmissible gastroenteritis coronavirus, J Virol. 1997 Apr; 71(4):3285-7.
- Zhou H, et al., Structural definition of a neutralization epitope on the N-terminal domain of MERS-CoV spike glycoprotein, Nat Commun. 2019 Jul 11; 10(1):3068.
- Chi X. et al., A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science. 2020, DOI: 10.1126/science.abc6952
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.