The global COVID-19 pandemic is in its third year. The effects of the pandemic have been far-reaching and are still being felt worldwide, affecting every country to varying degrees. Lockdowns, social distancing, travel bans, and vaccine rollouts have been widely reported news occurrences since the pandemic began.
An alarming trend in multiple countries has been waning vaccine efficacy, paired with the emergence of new variants with stronger transmission and immune escape abilities. There is an urgent need for new and innovative vaccine modalities that overcome current hurdles with vaccination programs.
The regulatory approval of CanSinoBio’s Covidencia™ Air has been a recent breakthrough in COVID-19 vaccine research. This is a first-in-class inhaled aerosol vaccine which, according to the company, induces comprehensive SARS-CoV-2 protection after a single breath.
Another inhaled aerosol vaccine swiftly followed the development of Covidencia™ Air. This vaccine was developed by the Indian firm Bharat Biotech and is intended for restricted use in emergencies. This article will explore what the development of these new, innovative technologies means for the future of vaccine research.
Routes of administration: Inhalation vs. Intramuscular
Conventional vaccine delivery is performed intramuscularly. These vaccines are designed to stimulate systemic immune responses in the patient. In doing so, they trigger neutralizing antibody production, such as IgGs. An inhaled aerosol vaccine is designed to simulate respiratory viral infection.
By simulating this type of infection, increased surface-level immune responses are stimulated, leading to a rapid immune response. Inhaled vaccines already exist in the form of nasal sprays, but initial studies have raised concerns about their limited efficacy.
The new class of inhaled aerosol vaccines has proven promising in recent research, as their biodistribution into the deeper respiratory tract compared to nasal vaccines is vastly improved.
Preliminary studies have demonstrated a higher immune response in inhaled aerosol vaccines due to their increased biodistribution. Therefore, they confer improved protective effects against infection with respiratory viruses such as COVID-19.
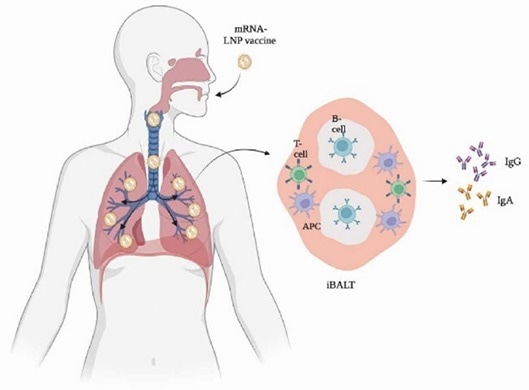
Figure 1. Mucosal immune response in the lung after vaccination through novel mRNA inhaled vaccine. 1 Image Credit: ACROBiosystems
Evaluating the safety and efficacy of inhaled vaccines
Inhaled aerosol vaccines have several advantages. A main benefit of these vaccines is in logistics, as they have lower doses, can be stored in conventional storage conditions, and are widely available. In resource-limited countries that struggle with establishing new vaccines, this is highly advantageous.
In conventional intramuscular COVID-19 vaccines, vaccine efficacy is evaluated by assessing the levels of neutralizing antibodies, with cellular immunity evaluated by cytokine secretion and antigen-specific T-cell response. In regulatory settings especially, the testing of novel vaccine modalities is still incomplete.
The proposed efficacy of inhaled aerosol vaccines is based on mucosal immune response stimulation alongside cellular and humoral responses. In recent studies, the mucosal immune response has been highlighted as a critical immune response that needs further evaluation.
Evidence for the production of both local IgAs and IgGs being triggered by mucosal immune system stimulation has focused research attention on this immune response pathway in respiratory virus infections.
IgAs are produced by mucosal tissues and are responsible for pathogen neutralization in respiratory infections. Due to this key contribution to the host’s immune response, IgAs have emerged as a target of interest when evaluating the efficacy of aerosol vaccines.
The next generation of vaccines
Inhaled aerosol vaccines, overall, represent a new tool in the clinical toolkit in the fight against respiratory diseases such as COVID-19. They do not require needles and can be administered without trained healthcare professionals. With lower vaccine doses needed, they are undoubtedly a significant breakthrough.
According to a London health analytics company, currently, around 100 inhaled aerosol-based COVID-19 vaccines are in development globally. Around 20 have reached the stage of human clinical trials.2
Table 1. Source: ACROBiosystems
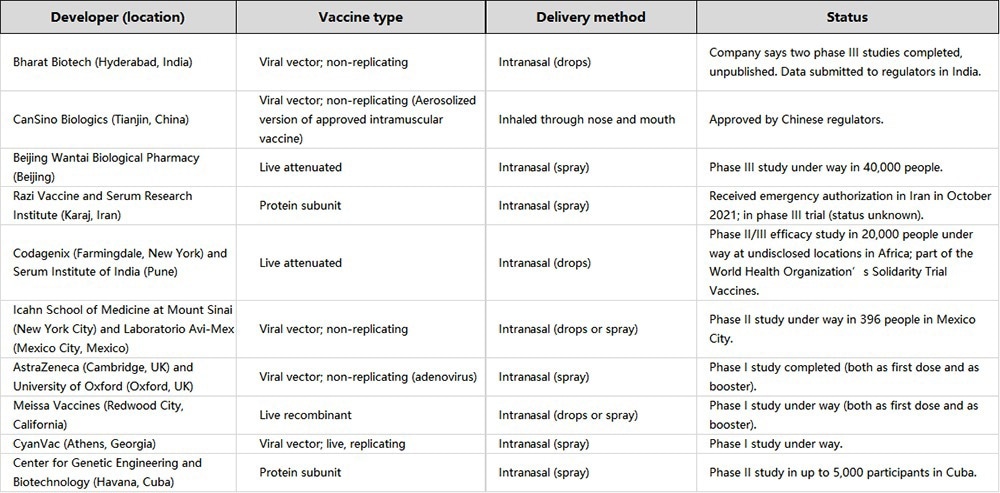
Despite the potential of inhaled aerosol vaccines, conventional intramuscular vaccines are projected to retain their front-line defense status for vaccination. These methods are more mature and provide stronger immune responses. Many companies are developing aerosol vaccines as a complement or enhanced booster.
This strategy is supported by a growing research interest in the concept of “prime-pull” vaccine approaches, where the immune system is primed by intramuscular vaccines, with inhaled aerosol vaccines acting as a booster to improve immune response, overcoming issues with waning vaccine efficacy.
The effectiveness and potential of inhaled aerosol vaccines for COVID-19 still need to be evaluated properly, mainly as more of these types of vaccines obtain approval for widespread use. In theory, they could significantly impact the COVID-19 pandemic, preventing infections.
By employing inhaled vaccines, the patient’s “first line of defense” against COVID-19 could be significantly boosted, and prevent onward transmission. As Mike Ryan, executive director of the World Health Organization’s Health Emergencies Programme said at a press briefing: “But it remains to be seen.”
ACROBiosystems commercial solutions for inhaled aerosol vaccines
ACROBiosystems is continuously monitoring the development of the COVID-19 pandemic and the vaccine research landscape.
To support the in-depth and supplemental characterization of inhaled aerosol vaccines, the company offers a series of IgA antibody titer detection kits across a range of variants and Ab isotype detection. Contact the experts today at ACROBiosystems today to find out more.
Table 2. Source: ACROBiosystems
| Lineage |
Cat. No. |
Product Description |
| Wild type |
RAS-T096 |
Anti-SARS-CoV-2 (Wild Type) Antibody IgA Titer Serologic Assay Kit (Spike RBD) |
| RAS-T098 |
Anti-SARS-CoV-2 (Wild Type) Antibody IgA1 Titer Serologic Assay Kit (Spike RBD) |
| RAS-T100 |
Anti-SARS-CoV-2 (Wild Type) Antibody IgA2 Titer Serologic Assay Kit (Spike RBD) |
| B.1.1.529 |
RAS-T097 |
Anti-SARS-CoV-2 (BA.1) Antibody IgA Titer Serologic Assay Kit (Spike RBD) |
| RAS-T099 |
Anti-SARS-CoV-2 (BA.1) Antibody IgA1 Titer Serologic Assay Kit (Spike RBD) |
| RAS-T101 |
Anti-SARS-CoV-2 (BA.1) Antibody IgA2 Titer Serologic Assay Kit (Spike RBD) |
| BA.2 |
RAS-T104 |
Anti-SARS-CoV-2 (BA.2) Antibody IgA Titer Serologic Assay Kit (Spike RBD) |
| RAS-T105 |
Anti-SARS-CoV-2 (BA.2) Antibody IgA1 Titer Serologic Assay Kit (Spike RBD) |
| RAS-T106 |
Anti-SARS-CoV-2 (BA.2) Antibody IgA2 Titer Serologic Assay Kit (Spike RBD) |
References and Further Reading
- Jansen E., Frijlink H., Ruigrok M., et al. (2022) Are Inhaled mRNA vaccines safe and effective? A review of preclinical studies. Expert Opin Drug Deliv. 19(11):1471-1485.
- COVID-19. https://www.airfinity.com/products/covid-19
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.