Experiments were conducted to establish a laser diffraction-based method for the determination of lactose powder particle size.
This was accomplished using a Bettersizer 2600 equipped with a fully automatic dry dispersion system. This device enabled researchers to study the particle size measurement of lactose powders systematically.
The impact of different dispersion pressures on the particle size distribution measurement was investigated, and a study into the precision of the dry dispersion method was undertaken. A comparison of measurement results revealed that dry dispersion can control risks well and improve data correlation.
The importance of excipients
An excipient’s powder properties have a significant effect on the preparation process and on the overall quality.
When selecting excipients for a tablet, its prescription and process design can be developed using the more traditional scientific and quantitative approach - measuring the excipient’s powder study index. Ensuring that excipients are properly quantitatively controlled can help to ensure the stability and quality of the tablets.
The USP provides clear regulations and guidance on the determination of raw material particle sizing using laser diffraction; for example, the instrument’s structure and principle, the specific approach to wet and dry dispersions and several factors should be taken into account throughout the measurement process.
Lactose remains one of the most frequently employed tablet excipients, but despite the overall level of specificity in the USP, there is no guidance on selecting dispersive pressure and effectively evaluating results from dry and wet methods when working with lactose.
The experiment detailed below involved systematic research on lactose particle size distribution measurement in line with requirements in ISO 13320 and the USP.
Experiment
Instruments
This experiment utilized a Bettersizer 2600 laser particle size analyzer from Bettersize Instruments Ltd. and an MS303S electronic scale from Mettler Toledo.
Sample and reagent
Two samples were employed for comparison:
- Non-micronized lactose sample (batch No.00116-17)
- Micronized lactose sample
Particle size distribution measurement and method evaluation
The dry dispersion method transports powder particles using compressed air, causing powder particles to disperse via airflow sheer, collision of particles with each other and collision of particles and pipe.
Lactose powder is typically comprised of small organic molecules particles prone to breaking under the stress of shearing and collision.
It is important to ensure that large agglomerates disperse without breaking the original particles. The USP requires an investigation into the impact of dispersive intensity on measurement results (USP 429) to be completed.

Image Credit: Bettersize Instruments Ltd.
In the dry dispersion experiments presented here, an investigation was conducted into the effect of dispersed pressure on particle sizing results. Dispersed pressure ranged between 0.05 MPa and 4 MPa, with tests performed every 0.05 MPa to 0.1 MPa.
The results are as follows:
- Abscissa: dispersion pressure
- Ordinate: particle size
- Blue curve: D10
- Orange curve: D50
- Grey curve: D90
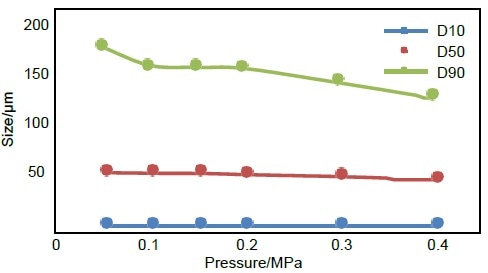
Figure 1. Particle size pressure titration data of No.2 micronized lactose sample. Image Credit: Bettersize Instruments Ltd.
In an ideal pressure titration curve, particle size gradually decreases as dispersion pressure increases, with the curve slowly reaching a stable period. Should the pressure continue to increase, the curve will continue to move downward.
This downward trend corresponds to the gradual dispersal of large agglomerates to single particles, with drug particles potentially breaking if the pressure is increased further.
In the pressure titration curve shown here, the lactose sample demonstrated a notable stable platform, illustrated by the results between 0.1 MPa and 0.2 MPa.

Figure 2. Particle image of No.2 micronized lactose sample. Image Credit: Bettersize Instruments Ltd.
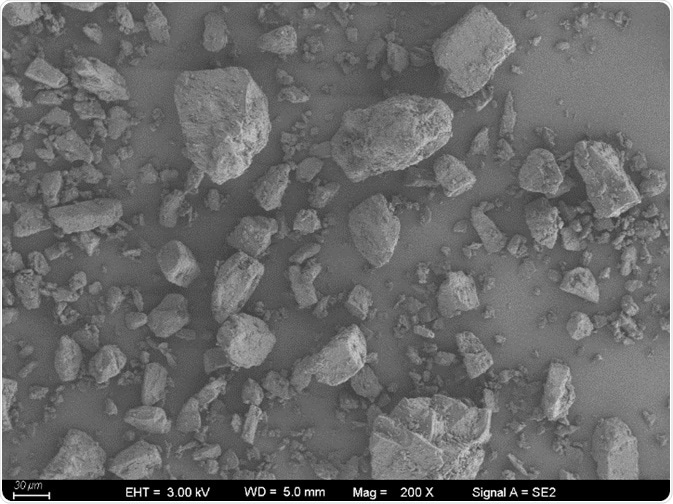
Figure 3. Particle image of No.2 micronized lactose sample. Image Credit: Bettersize Instruments Ltd.
For further investigation, images of micronized lactose samples (Figure 2 and Figure 3) were captured that confirmed the sample was comprised of semi-transparent irregular crystal particles. Therefore, the sample would be at increased risk of particle breakage during an increase in dry dispersion pressure.
Dry dispersion method precision
Since lactose is so fragile, the precision of the sample size was investigated under a dispersion pressure of 0.1 MPa. Figure 4 and Figure 5 display both the particle size distribution curves and repeatability data for the p-micronized and micronized samples.
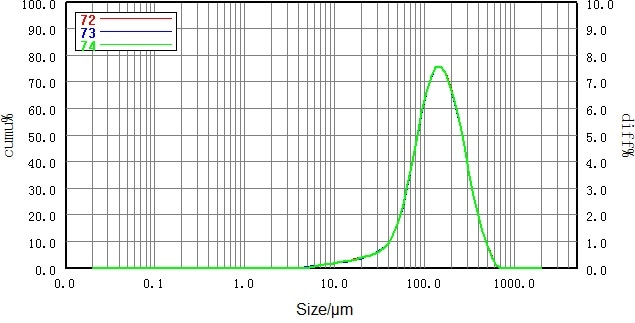
| Number |
Sample |
D10/μm |
D50/μm |
D90/μm |
| 72 |
No.1 lactose |
57.40 |
141.4 |
304.1 |
| 73 |
No.1 lactose |
57.15 |
141.2 |
304.0 |
| 74 |
No.1 lactose |
57.32 |
141.2 |
304.1 |
| RSD |
|
0.22% |
0.08% |
0.02% |
Figure 4. Particle size distribution and RSD of non- micronized lactose sample (dry dispersion). Image Credit: Bettersize Instruments Ltd.
Repeatability results for each sample confidently surpassed the requirements outlined in the USP: the fluctuation of the D50 data was under 0.5%, while the relative standard deviation of the D10 and D90 data was less than 1%, highlighting the excellent accuracy afforded by the dry dispersion method.
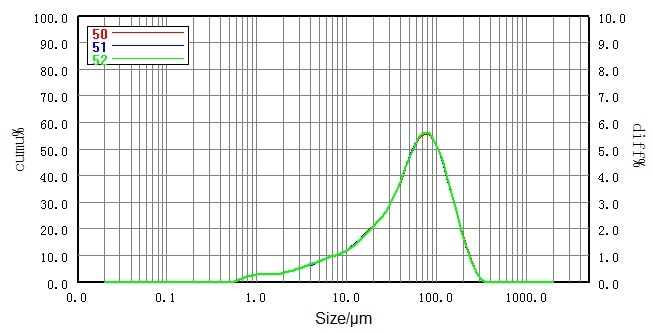
| Number |
Sample |
D10/μm |
D50/μm |
D90/μm |
| 50 |
No.2 lactose |
7.822 |
55.80 |
144.7 |
| 51 |
No.2 lactose |
7.840 |
55.76 |
144.0 |
| 52 |
No.2 lactose |
7.813 |
55.81 |
144.2 |
| RSD |
|
0.18% |
0.05% |
0.25% |
Figure 5. Particle size distribution and RSD of micronized lactose sample (dry dispersion). Image Credit: Bettersize Instruments Ltd.
Conclusion
Dry dispersion is an adaptive approach to measuring particle size distribution that is ideal for use with lactose.
The fragility of lactose does require the test pressure to be as low as possible, however, in order to avoid any breakage of the original lactose particles.
The experiment outlined here employed a pressure of 0.1 MPa and was able to achieve good results.
Acknowledgments
Produced from materials originally authored by Dr. Xuebing Li, Shiqi Liu, Mei Li, and Liyang Xu from Bettersize Instruments Ltd.
About Bettersize Instruments Ltd.

With over 25 years experience developing and manufacturing particle characterization instruments, Bettersize has introduced breakthrough technology in the field of particle size & shape measurement.
By achieving high quality and superior performance, our instruments provide precise analysis results of particle size, particle shape, and powder characteristics, helping scientists and engineers to understand material properties, facilitate research and improve production efficiency.
Bettersize product line for particle size and shape analysis includes instruments of all needs and budgets, from basic to advanced research models. These instruments are widely applied in Pharmaceuticals, Battery materials, Mining and minerals, Metals, Chemicals and Surface coatings, measuring materials with size ranges from nanometer to millimeter.
Focused on technology innovation, instruments manufacturing, application support and after-sales services, Bettersize provides expertise and professional solutions and assures customers the highest confidence in our products.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.