Thermo Fisher Scientific provides an extensive range of spectroscopy instruments and software, extrusion and rheological tools and service and support.
This allows pharmaceutical labs to secure quality and product safety while complying with regulatory standards throughout the drug development and formulation processes.
Chemical and biological must meet rigorous quality and regulatory requirements in order to develop drugs that are safe.
The Thermo Scientific spectroscopic and extrusion portfolio promotes the research work and facilitates quality and regulatory compliance throughout the drug formulation, development and manufacturing processes.
Thermo Scientific’s experienced team functions as a pharmaceutical lab partner to always assist its customer’s efforts. This is made possible due to the available selection of advanced spectrophotometers and software to guarantee compliance with the latest regulatory requirements.
In addition to spectroscopy and materials characterization tools, Thermo Scientific offers solutions in:
- Bioproduction
- Chromatography and mass spectrometry
- Assays, media and other consumables for drug discovery research
- Services and support for drug development, clinical trial logistics, commercial manufacturing • Clinical research
A comprehensive array of instruments
Thermo Scientific’s extensive portfolio includes X-ray plasmon spectroscopy (XPS), energy-dispersive spectroscopy (EDS), Raman, Fourier-transform infrared (FTIR), near-infrared (NIR), X-ray diffraction (XRD), rheometers and ultraviolet-visible (UV-Vis) spectroscopy.
Using advanced and innovative instruments, customers are guaranteed quality control across all steps and phases of the workflow, supplying drug product consistency from early formulations to large-scale production, utilizing rapid, non-destructive methods.
Thermo Scientific Pharmaceutical Extruders enable the production of consistent API dispersion in drug formulation labs, from initial research through clinical trials and production.
This is made possible with three different sizes of twin-screw extruders that present the possibility of hot melt extrusion (HME) and wet granulation (twin-screw granulation or TSG) capabilities.
When additional help or service is required, Thermo Scientific’s services and support teams can offer the repair of key instruments as well as after-sales service and support.
Data security to meet compliance requirements
Concerning software requirements, customers can secure laboratory data confidently, ensuring that it is compliant with data security regulations using Thermo Scientific’s latest software solutions.
Thermo Scientific Security Suite Software can be paired with several analytical software tools to supply the data integrity required to meet 21 CFR Part 11 regulations for electronic documents.
The Thermo Scientific™ SolstiX™ XRD Software with Security Suite package is readily available with the company’s complete ARL™ EQUINOX XRD range and permits 21 CFR Part 11 compatibility.
Many of Thermo Scientific’s instruments allow customers to take advantage of crucial compliance solutions built into the instrument software (for instance, the Thermo Scientific Evolution™ 350 UV/Vis Spectrophotometer includes 21 CFR Part 11, USP and PHEUR compliance).
The Thermo Scientific Nicolet™ Summit FTIR Spectrometers, NanoDrop™ One UV-Vis, Evolution UV-Vis and ARL EQUINOX XRD systems all promote endorsed configurations for pharmaceutical QA/QC that follow the strict 21 CFR Part 11 requirements.
Thermo Scientific OMNIC™ Paradigm Software gathers data, evaluates samples and produces workflows. All spectral data is saved in a central database, which offers increased data storage flexibility and security.
Many of these software solutions supply traceability with complete audit trails, always making sure the integrity of data is well-maintained throughout its life cycle.
From research to production, Thermo Fisher Scientific allows pharmaceutical labs to create safe and effective drugs that comply with stringent regulatory requirements.
By offering a complete spectroscopy and materials characterization tool set, intuitive data analysis software and one of the industry’s biggest service and support organizations, Thermo Scientific helps drug producers guarantee quality from start to finish.
Table 1. Source: Thermo Fisher Scientific – Materials & Structural Analysis
Comprehensive, full-spectrum support
When purchasing instrumentation from Thermo Fisher Scientific, customers are introduced to a partnership with an experienced team who they can count on for assistance throughout the workflow.
Thermo Scientific delivers comprehensive, worldwide application support and training, after-sales customer service, instrument compliance solutions and an international, world-class support network.
Thermo Scientific can use the combined experience of its entire organization to assist customers in the successful navigation of the drug discovery and development environment to distinguish, assess and obtain regulatory approval for marketing a drug product.
Thermo Scientific is on hand to support its customers along the complex pathway, no matter how challenging, from R&D through to preclinical studies and clinical trials, to the final review and approval of a commercial product.
Unique capabilities
How do the Thermo Scientific spectroscopic analytical instruments and services optimize the pharma/biopharma workflow?
Thermo Scientific’s instruments provide dependable, stable performance with great reproducibility. Its team can help customers select, use and service their tools to guarantee drug quality, safety and speed to market.
When paired with cutting-edge software, an instrument can capture the data required throughout the workflow to meet quality needs. This includes 21 CFR Part 11 compliance, GMP guidelines, pharmacopoeia (USP and EP) requirements while producing the reports necessary for regulatory filings.
Each qualified installation, in cooperation with world-leading technical service and support experts, sets the stage for a successful journey through the workflow. The technology and method that customers choose can help release analytical benefits at each stage of the process:
Table 2. Source: Thermo Fisher Scientific – Materials & Structural Analysis
| Technology |
Benefit example |
| FTIR |
- Contaminant identification
- Identify molecular polar substructure
- Couple with Raman to assess molecular structure and stereochemistry
|
| NIR |
- Inline monitoring of extrusion process
- As a process analytical tool (PAT), allows real-time monitoring of product quality attributes to help deliver a constant desired product quality
- Final product quality control in QC lab
|
| Raman |
- Can map areas of a sample (e.g., tablet) to screen for component distribution or contamination
- Reveal subtle structural and orientation differences in molecules
- Facilitate quantitative analysis at micro levels
|
| UV-Vis |
- Minimize sample
handling
- Assess concentration and purity in small or large molecule formulations
- Micro UV spectrometry offers a large dynamic range to eliminate dilution
|
| Rheometry |
- Measure critical rheological properties of compounds and extrusions
- Use with polarization microscopy to study crystallization behavior to manage compounding and extrusion
- Efficiently screen delivery parameters
|
| XRD |
- Ensure product safety with the ability to assess polymorphs and amorphous content
- Speed up analysis time for organic samples
- Measure samples in reflection or transmission mode in the same configuration
|
| HME |
- Enhance drug characteristics, including solubility enhancement, drug stability, consistent API dispersion, taste-masking, specialized dosing forms, and reformulation
- Shorten the path from feasibility studies to production
- Enable easy implementation of in-line monitoring, reduction of offline sampling, and minimization of reagents and disposables
|
| TSG |
- Facilitates implementation of continuous manufacturing
- Use the hybrid mode to run HME and TSG
|
Software
solutions |
- State-of-the-art software with a dedicated development team following updates in regulations requirements, full audit trails
- Unified data security suite across product lines
- Homogenized IT administrative tasks with a common security framework
|
Solutions for scaling-up and partnering instruments
Thermo Scientific’s team of pharmaceutical experts will assist its customers and help them achieve product development goals.
They can also help customers select the right instrument for any given workflow, whether just starting or ready to move from the pilot-scale in research and discovery to the small-to-medium scale batches needed in the development and clinical study phases and ultimately to commercial-scale manufacturing with continuous processes.
For granulation and extrusion, Thermo Scientific’s support experts can help customers make the best choices on size and compatibility with upstream and downstream processes that satisfy formulation needs at all research, development and production stages.
When working through the feasibility studies phase, the Thermo Scientific™ Pharma mini HME Micro-Compounder can conduct rapid and early assessment of new API/excipient formulations and help determine drug candidates for hot melt extrusion by combining as little as three grams of material.
When customers are ready to scale up, especially when in possession of a promising, new API that cannot otherwise be solubilized, Pharma 11, Pharma 16 and Pharma 24 twin-screw extrusion technology can help overcome such obstacles.
Finally, when customers are ready to streamline manufacturing, Thermo Scientific are on hand to help seamlessly integrate in-line analysis equipment and accessories upstream (like feeders) and downstream (like dryer, coating and forming technology) for an improved, continuous production process.
Table 3. Source: Thermo Fisher Scientific – Materials & Structural Analysis
| |
 |
 |
 |
 |
Twin-Screw
Extruders |
Pharma mini
HME |
Pharma 11 |
Pharma 16 |
Pharma 24 |
| Recommended for |
- Feasibility
- Small-scale HME
- Implants (ophthalmic and injectable)
|
- Research and discovery
- Laboratory scale
|
- Development pilot scale
- Production scale
|
- Manufacturing
- Production scale
|
| Suitable for |
- Co-extrusion
- Implant production
|
- Co-extrusion
- Sheet extrusion
- Implant production
|
- Co-extrusion
- Sheet extrusion
- Implant production
|
- Sheet extrusion
- High-volume HME production
|
| Downstream options |
|
|
- Conveyor belt
- Pelletizer
- Chill roll
|
- Conveyor belt
- Pelletizer
- Chill roll
|
| Typical throughput HME** |
3 g batch
or 100 g/h |
20 g/h –
2.5 kg/h |
0.5 kg/h –
10 kg/h |
1 kg/h –
30 kg/h |
| Typical throughput TSG** |
* |
Up to
3 kg/h |
Up to
20 kg/h |
Up to
80 kg/h |
Unit
type |
Benchtop
model |
Benchtop
model |
Floor
model |
Floor
model |
| Screw design |
Conical, co-/counter-rotating |
Parallel, co-rotating |
Parallel, co-rotating |
Parallel,
co-rotating |
* No TSG option
* Depending on formulation
* All parallel screw designs are interchangeable between HME and TSG operation
All extrusion instruments are made of pharma-grade steel and allow you to meet GMP compliance standards.
The workflow
Table 4. Source: Thermo Fisher Scientific – Materials & Structural Analysis
Research
and
discovery |
Development |
Clinical study |
Manufacturing |
| Identify & validate target of interest and identify leads |
Advance a lead through testing, formulation & synthesis scale-up |
Experiment & observe to generate safety & efficacy data |
Industrial-scale synthesis of pharmaceuticals |
| FTIR, LC-MS, Micro UV, Raman, SPM, UV-Vis, XRD |
FTIR, HME, NIR, Raman, TSG, XRD |
FTIR, HME, NIR, Raman, TSG |
FTIR, HME, IR microscopy, NIR, NMR, Raman, TSG, UV-Vis, XRD |
| High-end instrumentation gives you the edge to discover your next breakthrough in a shorter timeframe |
Multi-purpose equipment enables you to develop the right analytics to ensure your drug quality and performance |
Reliable equipment and analyzers empower you to confidently produce your small-to-medium batches |
Highly automated workflows and analyzers help you safeguard your process from incoming material through production and final product ID |
❱ Simultaneous analysis of multiple components
❱ Discover pioneering solutions faster
❱ Comply with quality and regulatory standards
Step 1: Research and discovery
Basic research and feasibility study methods motivate an understanding of the underlying disease mechanism while identifying the therapeutic target and facilitating appropriate selection of likely drug candidates for that target to progress to the next step in the process.
Discovery integrates assay development and high-throughput screening methods to advance the identification, analysis and characterization of the most promising small molecule and biotherapeutic compounds.
It also assesses how the candidates act in testing in vitro and in vivo with drug, metabolism and pharmacokinetic (DMPK) studies and subsequently choosing how to appropriately formulate the drug for delivery.
Table 5. Source: Thermo Fisher Scientific – Materials & Structural Analysis
| The process of identifying and validating the target of interest and identifying potential lead compounds. |
| TSG/HME |
Raman |
FTIR |
XRD |
UV-Vis |
Micro UV |
| Produce extruded and granulated formulations |
- Perform rapid, quantitative analysis at micro levels with minimal sample preparation
- Equipment with open architecture
- Streamline material analysis with OMNIC Paradigm Desktop Software
|
- Streamline material analysis with OMNIC Paradigm Desktop Software
- Simplify data collection and rely on the security of database storage
- Acquire spectra from far-infrared to visible
|
- Identify polymorphs
- Validate crystalline structure and amorphous content
- Non-destructive analysis
- Suitable for very small quantities
|
- Count on accurate, reliable performance
- Meet quality and 21 CFR Part 11, USP, EP compliance with Evolution 350 UV-Vis software
|
- Minimize sample
handling
- Measure highly concentrated analytes
- Meet quality and 21 CFR Part 11 compliance with NanoDrop One UV-Vis software
|
Table 6. Source: Thermo Fisher Scientific – Materials & Structural Analysis
| Method/application |
Spectroscopy tools |
| Understanding the disease mechanism |
LC-MS, Raman, UV-Vis |
| New chemical synthesis |
FTIR, MS, UV-Vis |
| Biomolecule analysis (proteins, peptides, nucleic acids) |
Micro UV, UV-Vis, FTIR, Raman |
Material characterization (physicochemical properties,
secondary structure and conformation, crystallography/crystallinity,
API domain, morphology) |
UV-Vis, EM, XRD, SPM, Raman |
| Assay development |
FTIR, Raman, NIR, UV-Vis |
| Process development |
MS, NMR, NIR, HME, TSG, Raman |
| Crystallography |
XRD |
| DMPK studies |
Raman, UV-Vis |
Step 2: Development
This step moves into pilot-scale development of the drug, tackling the analytical methods, process chemistry, process development and optimization and approaches for scale-up.
Early development also embraces the formulation process to evaluate the optimal composition and compounding method of the active pharmaceutical ingredient (API) and other components necessary for safe, timely and effective delivery to the therapeutic target.
Table 7. Source: Thermo Fisher Scientific – Materials & Structural Analysis
| The process for advancing a lead compound from discovery to clinical trial through testing (pharmacokinetics, toxicology, and more), formulation, and synthesis scaleup. |
| TSG |
NIR |
Raman |
FTIR |
XRD |
HME |
| Produce extruded and granulated formulations |
- Monitor and analyze the workflow inline
- Quality control process and method development
|
- Assess bioavailability, molecular structure, and stereochemical conformation of formulation and corrections
- Visualize API/excipient distribution
- Streamline material analysis with OMNIC Paradigm Desktop Software
|
- Analyze powders and liquids directly
- Streamline material analysis with OMNIC Paradigm Desktop Software
- Simplify data collection and rely on the security of database storage
|
- Identify and investigate all polymorphs
- Assess API stability with aging/temperature & humidity
- Meet GMP recommendations and 21 CFR part 11 requirements
|
- Enhance solubility of API
- Create consistent dispersion of API
- Address stability, taste, dosage form, and other characteristics
|
Table 8. Source: Thermo Fisher Scientific – Materials & Structural Analysis
| Method/application |
Spectroscopy tools |
| Formulation design and optimization (proportion of ingredients, polymer composition) |
XRD, FTIR, HME, Raman, TSG |
| Drug delivery systems (injectable implant, tablet, powder, softgel, nanoparticle, orally disintegrating films, patches, other) and dosage forms (solid oral, transdermal, transmucosal, subcutaneous, buccal) |
FTIR, HME, TSG |
| Coating analysis |
Raman, FTIR microscopy, NIR |
| Solubility enhancement |
HME |
Timed delivery enhancement (controlled, extended, sustained,
delayed release) |
HME, TSG |
| Compounding |
HME, FTIR |
| Extrusion (coextrusion, hot melt extrusion) |
HME |
| Granulation (twin-screw, wet, dry, continuous)) |
TSG |
| Continuous manufacturing |
NIR, Raman, HME, TSG |
| Dispersion analysis (amorphous solid dispersion, crystalline solid dispersion) |
XRD |
| Stability testing |
Raman, XRD |
| Raw material testing |
FTIR, XRD |
| API distribution |
Raman |
| Polymorphism analysis |
XRD, Raman |
Step 3: Clinical study
This step covers the typical Phase I, Phase II and Phase III clinical trial aspects with human participant groups in increasing scale.
While Thermo Scientific’s instrument portfolio has been designed for research use and not mapped to methods or applications for use with human samples, some of its instruments can be useful when adjusting the upcoming manufacturing process to optimize solubility and bioavailability of the API (HME), or to manage the integration of continuous manufacturing (TSG).
Table 9. Source: Thermo Fisher Scientific – Materials & Structural Analysis
| The process of doing experiment or observations done in clinical research to generate data on safety and efficacy. |
| TSG |
Raman |
FTIR |
HME |
- Produce extruded and granulated formulations
- Implement continuous manufacturing
- Reduce human error and enhance productivity
|
- Profile drugs in living cells
- Expect stable performance with minimal downtime
- Couple and customize to almost any equipment with open architecture
|
- Simplified data collection
- Achieve high-quality results on small samples
- Can acquire spectra from far-infrared to visible
|
- Enhance solubility of API
- Process scale-up
|

Image Credit: Thermo Fisher Scientific – Materials & Structural Analysis
Table 10. Source: Thermo Fisher Scientific – Materials & Structural Analysis
| Method/application |
Scope |
| Phase I – safety, tolerability, pharmacokinetic (PK) studies |
Assess safety in a small population of healthy humans |
| Phase II – dose range finding, early side effects, GMP formulation |
Assess therapeutic effects in midsized population of healthy and diseased humans |
| Phase III – large-scale safety, efficacy studies |
Assess long-term effects in larger population of humans. |

Image Credit: Thermo Fisher Scientific – Materials & Structural Analysis
Step 4: Manufacturing
This step addresses the process scale-up that takes place in industrial-scale manufacturing, as well as the tasks associated or involved with surrounding procedure and production.
Table 11. Source: Thermo Fisher Scientific – Materials & Structural Analysis
| Industrial-scale production of pharmaceutical drugs: hot-melt extrusion, milling, tablet pressing, coating, etc. |
| TSG |
NIR |
Raman |
FTIR |
XRD |
UV-Vis |
HME |
IR Microscopy |
| Produce extruded and granulated formulations |
- PAT
- QA/QC
- Monitor and analyze component mixing inline
- Test finished products
- Meet quality and 21 CFR Part 11 compliance with ValPro package and OMNIC Security Suite software
|
- Test finished products
- QA/QC
- Reduce operator variability
- Meet quality and 21 CFR Part 11 compliance with ValPro package and OMNIC Security Suite software
|
- Test finished products
- QA/QC
- Analyze packaging material
- Simplify data collection and rely on the security of database storage
- Meet quality and 21 CFR Part 11 compliance with ValPro package and OMNIC Security Suite software
|
- Test finished products
- QA/QC
- Identify polymorphs
- Take transmission and reflection measurements in the same configuration
- Meet quality and 21 CFR Part 11 compliance with ARL EQUINOX XRD system software
|
- Assess sample quantity and purity
- Meet quality and 21 CFR Part 11, USP, EP compliance with Evolution 350 UV-Vis software
|
- Process scale-up
- Create new dosage forms and delivery systems
|
- Analyze final packaging material
|
Table 12. Source: Thermo Fisher Scientific – Materials & Structural Analysis
| Method/application |
Spectroscopy tools |
| Quality checks (raw materials, finished goods, performance qualification consultation, batch and lot analysis, QA/QC) |
FTIR, XRD, NIR, UV-Vis, Raman |
| Process control (reaction monitoring, bioreactor monitoring, feedback/feed-forward/real-time monitoring, fluid bed drying/moisture/residual solvent analysis) |
FTIR, NMR, NIR, XRD |
| Regulatory compliance (USP/PHEUR, FDA, data security, informatics, traceability, auditing trails, analytical instrument maintenance, calibration, and certification, IQ/OQ/PQ qualification consultation) |
OMNIC security suite software and ValPro Package |
| Solubility enhancement of API |
HME with Raman |
| Continuous manufacturing |
TSG |
| Inline analysis of component mixing |
NIR |
| Packaging material analysis |
IR microscopy, Raman microscopy |

Image Credit: Thermo Fisher Scientific – Materials & Structural Analysis
Overarching quality from start to finish
Quality control and assurances during the pharma process include routines to help determine potential drug contaminants, guarantee product consistency and safety and maintain regulatory compliance.
Thermo Scientific’s instruments not only develop the drugs but also assist in the analysis and recording of data from inbound and raw materials through outbound and finished goods across the complete workflow process.
Its team can supply excellent service and support critical, making sure downtime remains minimal throughout the process and when a customer is ready to release a batch or crucial product.
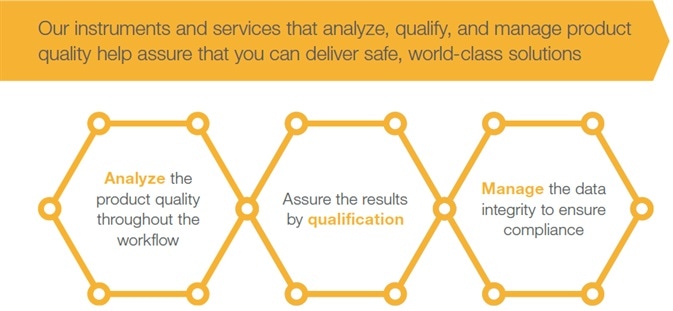
Image Credit: Thermo Fisher Scientific – Materials & Structural Analysis
Table 13. Source: Thermo Fisher Scientific – Materials & Structural Analysis
| Application |
Before
you
begin |
Research
and
discovery |
Development |
Clinical
study |
Manufacturing |
Inbound and raw
material analysis |
|
● |
● |
|
● |
Outbound and
finished goods
analysis |
|
● |
● |
|
● |
| Data integrity |
|
● |
● |
● |
● |
| Traceability |
|
● |
● |
● |
● |
Minimize sample
handling |
|
|
● |
|
● |
Sample purity/
contamination |
|
● |
● |
● |
● |
| Minimize downtime |
|
● |
● |
● |
● |
| Stable performance |
|
● |
● |
● |
● |
| Service and support |
● |
● |
● |
● |
● |
Surrounded by support
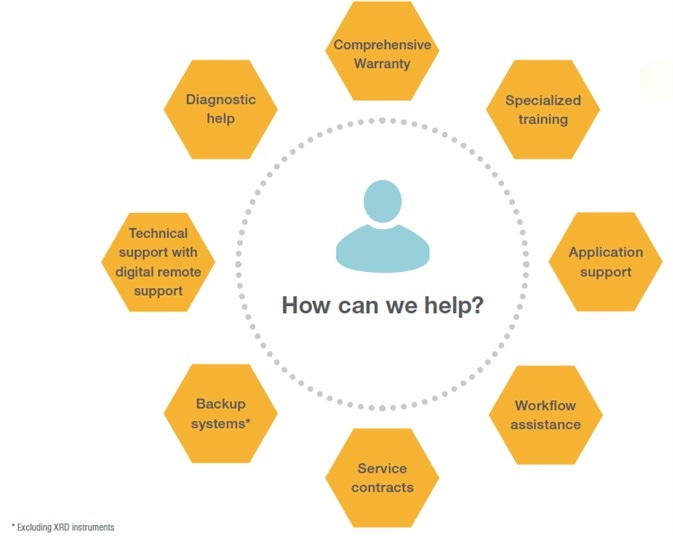
Image Credit: Thermo Fisher Scientific – Materials & Structural Analysis
Thermo Fisher Scientific’s instruments and services analyze, qualify and manage product quality, helping assure customers in the delivery of safe, world-leading solutions:
- Analyze the product quality throughout the workflow
- Assure the results by qualification
- Manage the data integrity to ensure compliance
Thermo Fisher Scientific recognizes the importance of instrument uptime in pharmaceutical QA/QC labs. Therefore, it offers an extensive range of service offerings to guarantee timely response and repair of mission-critical instruments.
Thermo Fisher Scientific’s comprehensive, global after-sales service and support, including instrument installation, applications support, on-site technical consultancy, instrument qualification, and compliance services.
Thermo Scientific’s customers can also take advantage of its premier offering, which includes a two-day on-site response or industry-exclusive no-charge requalification guarantee when adding OQ to a qualifying plan - hassle-free help with audit readiness for GMP compliance.
Additionally, with a global team of expert consultants, Thermo Scientific can also help its customers understand 21 CFR Part 11 and pharmacopeia requirements as they navigate complex industry demands.
Service
Make use of Thermo Scientific’s extensive expertise to reach the potential of discovery.
With a global business infrastructure and experienced key account management team, Thermo Scientific can offer outstanding post-sale support to follow instrument installation.
This is conducted by certified engineers who are on hand to respond to customer requests for calibration service, routine maintenance, corrective maintenance repairs, emergency response and other warranty and post-warranty service contracts.
Thermo Scientific also has instructors who have developed and used the product, developed the applications and methods and maintained the instruments to help train customer teams.
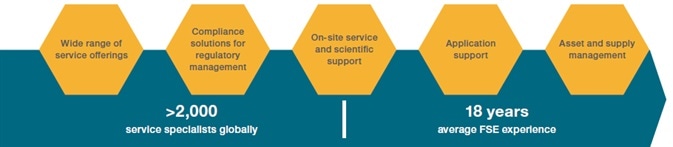
Image Credit: Thermo Fisher Scientific – Materials & Structural Analysis
About Thermo Fisher Scientific – Materials & Structural Analysis
 Thermo Fisher Materials and Structural Analysis products give you outstanding capabilities in materials science research and development. Driving innovation and productivity, their portfolio of scientific instruments enable the design, characterization and lab-to-production scale of materials used throughout industry.
Thermo Fisher Materials and Structural Analysis products give you outstanding capabilities in materials science research and development. Driving innovation and productivity, their portfolio of scientific instruments enable the design, characterization and lab-to-production scale of materials used throughout industry.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.