The concept of cultivated meat (also referred to as cell-based or cultured meat) has existed for about 20 years, with the first cultivated tissue created in 2000–2001 by two different academic research groups.
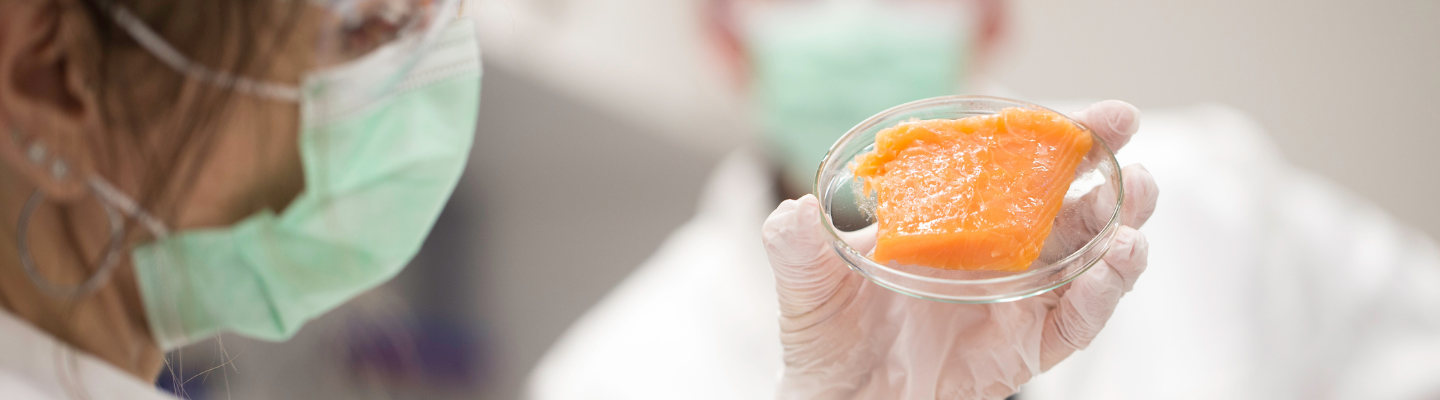
Image Credit: Hamilton Company
NASA funded one research group and was seeking a way to produce muscle protein for astronauts who were undertaking long-term space travel, while the other group consisted of “bioartists”.1
As meat is made of cells, the focus is on cell culture manufacturing, which entails essentially the same processes that are widely employed for the manufacture of monoclonal antibodies (mAbs) or stem cell therapies.
Instead of expressing a drug substance from cultured cells like mAb production, the aim is to produce the correct types of cells in the correct quantities. This is similar to what is involved in stem cell applications.
The cultured meat is created in a bioreactor by utilizing living cells extracted from the relevant animal species in culture media and supplied with the appropriate feeds and supplements to support the growth and maintenance of the viability and health of the cells.
The challenge is to package the manufactured cells to look (and taste) like animal-based hamburger, steak, or sushi products to meet consumers' expectations and do so at a competitive price.
Two decades ago, the first cultivated meat hamburger cost approximately $330,000.2 By early 2022, the price had significantly reduced to $9.80, but cultivated meat continues to be more costly than animal-based meat, with the average production cost of an animal-based hamburger worldwide being approximately $2.2
The benefits of cultivated meat
The production of meat (beef, poultry, and fish) via cell culture has psychological and technical benefits. It does not require the raising (and ultimate killing) of large quantities of livestock in small spaces. This avoids the mistreatment of animals as well as the risk of diseases that may impact humans.
The environmental impacts of animal waste and greenhouse gases are also minimized, and antibiotic use is not required.
Production using controlled bioprocesses provides the perfect meat every time due to the same type of cells being generated throughout the manufacturing run.
This enables specialty meats to be obtained anywhere worldwide instead of only in limited locations where certain animals or fish are raised.
Recent regulatory developments
A key challenge encountered by the cultivated meat industry was recently partly addressed when the FDA (Food and Drug Administration) reported in March 2023 that it had completed a secondary pre-market consultation for a human food produced from cultured animal cells by GOOD Meat.
GOOD meat is a firm that uses living cells from chickens to manufacture cultured animal cell food.3
Although this voluntary pre-market consultation was not an approval process, it did involve evaluating the production process, including establishing cell lines and cell banks, manufacturing controls, and all inputs and components.
The FDA found no issues from this consultation, which is a significant first step toward the production and sale of cultivated food products being allowed in the United States. At present, only Singapore allows the sale of cultivated meat.
To be sold in the US, human food that is produced from cultured animal cells must meet the exact strict FDA requirements as other food, including applicable safety requirements and facility registration.
Any company that manufactures cultivated meat products is also required to pass a facility inspection by the United States Department of Agriculture's Food Safety and Inspection Service (USDA-FSIS) prior to entering the US market.
As the commercialization of cultivated meat products nears, the FDA works closely with USDA-FSIS to guarantee that the products are safe and accurately labeled.4 The two agencies jointly regulate any food produced from cultured cells of livestock and poultry under a formal agreement that was established in March 2019.5

Image Credit: Hamilton Company
New market driven by startups but facing scale-up challenges
Interest in cultivated meat has grown over the last 20 years, with many startups entering the space and slowly progressing from research and development to the pilot stage.
One study identified more than 150 companies globally that were developing cultivated meat products.6 However, none of these have realized commercial-scale production, as scale-up is a considerable challenge — even more so than the scale-up of a mAb process, which continues to be an ongoing challenge.
Biopharmaceutical processes create high-margin products that cover the high costs related to cell culture process development and implementation. However, the margins in the food industry are minimal, making it challenging to recover the costs of cultured meat production.
As a result, scale-up must be achieved in a way that substantially reduces the cost of cell culture.
Process optimization demands effective process analytical technology
Enabling successful scale-up initially demands the development of optimized processes that perform consistently and robustly by using effective process control measures. Ideally, optimization will facilitate processes to be developed that will behave similarly across scales.
Many scientists employed by cultivated meat developers are from the biopharmaceutical industry. They apply their knowledge and experience to optimizing cell culture processes to produce cultivated meat.
These scientists are employing design-of-experiment (DoE) strategies for the identification of the relevant critical process parameters (CPPs) and critical quality attributes (CQAs).
All this work requires access to real-time process data to guarantee that the cells in the bioreactor are within the optimal environment for the duration of the process. The cells require sufficient glucose and oxygen, the correct pH, and carbon dioxide to be removed.
Process analytical technology (PAT) makes the monitoring — and ultimately control — of these parameters feasible. Sensors for tracking pH, CO2 concentration, and dissolved oxygen (DO), as well as viable cell density (which is a key indicator of cell health), are crucial.
Minimal margins demand automation
Due to the low margins associated with the food industry, the automation of processes by using PAT and in-line sensors is fundamental to the success of cultivated meat production.
Combining in-line sensors with a feedback loop for the automated adjustment of process conditions (such as pH, DO, and nutrient addition) minimizes personnel demand while ensuring controlled and robust processes.
Innovative and intelligent sensor technologies from Hamilton
Hamilton Company offers advanced in-line sensors for many biotech applications. The innovative and intelligent sensors are the first that do not demand an external transmitter, making their installation and use easier.
These sensors can be incorporated into automated loops, as well as monitor their own performance, delivering confidence in the data they generate.
In the cultivated meat industry, one of the biggest challenges faced regarding scaling is the unexpected accumulation of CO2, which causes reduced cell viability. This leads to less biomass, and subsequently, less cultured meat product. (In biopharma the emphasis is on DO measurements.)
Hamilton has effective solutions for these processes, such as in-line CO2 monitoring, and it is vitally important for designing processes to have the ability to be seamlessly scaled and deliver the same level of productivity in 2-liter, 100-liter, and 2000-liter bioreactors.
The capacity for monitoring viable cell density in real-time is particularly important, as this measurement is typically conducted offline for biopharmaceutical applications.
However, in the cultivated meat industry, obtaining accurate measurements of viable cell density throughout the culture process is essential, as the cells are the product.
The Incyte sensor from Hamilton delivers real-time viable cell density data and a range of other information concerning cell behavior, facilitating the improved regulation and control of cultivated meat production processes.
In particular, the sensors measure the permittivity of the cells at various frequencies, and the obtained data may be correlated with texture and other attributes of cultivated meat products.
In general, it is feasible to monitor the quality of the biomass, and this is beneficial for guaranteeing optimum products but also for carrying out DoE experiments to identify less expensive media and feeds to help reduce production costs.
Hamilton is a one-stop-shop supplier of intelligent sensors for bioprocessing, and the company works closely with biotech manufacturers and bioreactor vendors.
Hamilton’s full range of sensors is designed to support all types of cell culture and fermentation, as well as other bioprocesses. The range can be integrated into top-quality single-use and stainless-steel bioreactors from leading equipment vendors to allow automated process control.
If the challenges surrounding scale-up and the associated funding and cost can be resolved, the growth potential of the cultivated meat market is substantial. Even if cultivated meat products account for only 10% of the entire meat production market, this would represent an entirely new, multi-billion-dollar industry.
References and further reading
- Stephens, Neil, Alexandra E. Sexton and Clemens Driessen. “Making Sense of Making Meat: Key Moments in the First 20 Years of Tissue Engineering Muscle to Make Food.” Front. Sustain. Food Syst. 10 Jul. 2019, Sec. Sustainable Food Processing.
- Bandoim, Lana. “Making Meat Affordable: Progress Since The $330,000 Lab-Grown Burger. Forbes. 8 Mar. 2022.
- FDA Completes Second Pre-Market Consultation for Human Food Made Using Animal Cell Culture Technology. U.S. Food and Drug Administration. Constituent Update. 21 Mar. 2023.
- “Human Food Made with Cultured Animal Cells.” U.S. Food and Drug Administration. Constituent Update. 21 Mar. 2023.
- Formal Agreement Between FDA and USDA Regarding Oversight of Human Food Produced Using Animal Cell Technology Derived from Cell Lines of USDA-amenable Species. U.S. Food and Drug Administration. 7 Mar. 2019.
- State of the Industry Report: Cultivated meat and seafood. Good Food Institute. 2022.
About Hamilton Company
Hamilton Company specializes in the development, manufacturing and customization of precision measurement devices, automated liquid handling workstations, and sample management systems. Hamilton's processes are optimized for quality and flexibility. Whether it's a custom needle with a quick delivery time frame, a special length pH sensor, or a comprehensive solution to fully automate your assay workflow, trust that Hamilton products will always meet your needs.
Hamilton Company has been a leading global manufacturer for more than 60 years, with headquarters in Reno, Nevada; Franklin, Massachusetts; Timișoara, Romania; Bonaduz, Switzerland; and subsidiary offices throughout the world.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.