Skip to:
Antibodies are an indispensable tool for the diagnosis, and treatment of several diseases. High affinity and extraordinary specificity for their target antigens are some properties desirable properties of antibodies.

Camelids are members of the biological family Camelidae, the only currently living family in the suborder Tylopoda. The extant members of this group are: dromedary camels, Bactrian camels, wild Bactrian camels, llamas, alpacas, vicuñas, and guanacos. Image Credit: Yasser El Dershaby / Shutterstock
Issues surrounding antibody-based therapeutics
Although antibodies are an attractive therapeutic option, the risk of immunogenic responses poses a major challenge. Immunogenic responses impact both safety and pharmacokinetic properties of the drugs, which ultimately affects the drug’s utility and efficacy. Additionally, the size of full-length antibodies (~150 kDa) prevents deep tissue penetration and can decrease efficient targeting of key sites.
Discovery of techniques to reduce the immunogenic potential while maintaining the bioactivity of the antibody molecule has been an important subject of biomedical research. Size minimization has become an important strategy to reduce the immunogenicity caused by antibodies while conserving the antigen–antibody interaction site. The strategy also leads to improvement in pharmacokinetic and pharmacodynamic characteristics of the therapeutic antibody.
Camelids are members of the biological family camelidae, which include animals like camels, dromedaries and llamas. The camelidae family is associated with a peculiar property of producing antibodies that lack the L (light) chains. Ever since the discovery that camelids produce such antibodies, they are being studied for a plethora of medical applications such as drug delivery, biosensors and conditions such as cancer, inflammatory and neurological diseases.
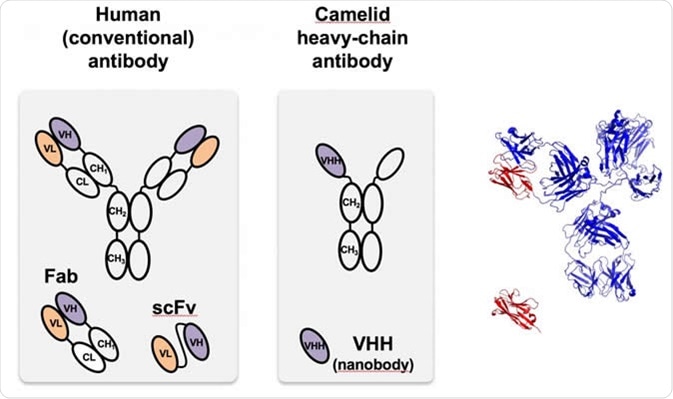
The camelid nanobody (center), first identified in camels, is a heavy-chain antibody that is much smaller and easier to program than antibodies found in most organisms, including humans, like that at left. At right, the monomeric camelid (red) is compared with the structure of the full-sized human antibody. VHH is a nanobody designed to target green fluorescent proteins used in proof-of-principle tests at Rice. Image Credit: Segatori Research Group/Rice University
The camelid antibodies, referred to as heavy chain-only antibody (HCAbs), are molecules with a molecular weight of around 90 kDa. HCAbs comprise nearly 10%–80% of the total immunoglobins (IgG) produced by camelids. The epitope binding site of HCAbs is composed of variable domain known as the single-domain antibody (VHH) or nanobody (Nbs). VHH is the antigen recognition site of HCAbs with extraordinary antigen-binding properties.
What are the benefits of camelid antibodies?
Despite their small size (~15 kDa), VHH or Nbs are associated with some exquisite characteristics:
VHH display high affinity and specificity to target antigen (comparable to conventional antibodies). They also have the unique ability to target antigenic epitopes at locations which are difficult to access by conventional antibodies because they are smaller in size. They can also penetrate more effectively into tissues.
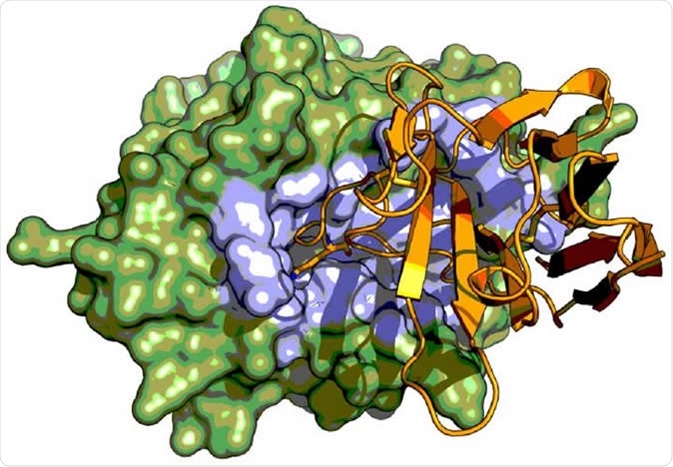
X-ray crystal structure serine protease urokinase-type plasminogen activator (green) in complex with a Camelid antibody fragment (orange). The Camelid antibody fragment display an unusual inhibitory mechanism by binding to the active site region (highlighted in blue) of the serine protease where it mimics the binding of substrate. Image Credit: Tobias-Kromann-Hansen
High solubility and physical stability - Due to their small size, single domain nature, and presence of hydrophilic amino acids, VHHs have high solubility and physical stability. These properties allow them to withstand harsh formulations and environments.
HCAbs also have flexible modularity that enables the production of recombinant protein with diverse properties. The small size and presence of a single-domain in VHH allows flexible engineering of Nbs. This, in turn, facilitates the chemical conjugation of proteins or drug molecules. This relative ease of genetic engineering leads to relatively low production cost.
Camelid antibodies in treating allergy
Type I hypersensitivity are allergic reactions caused by exposure to specific allergens. The role of immunoglobin E (IgE) in type I hypersensitivity is well established. Upon crosslinking by allergens, IgE bind to FcεRI, which are high-affinity receptors that control the activation of mast cells and basophils and participate in IgE-mediated allergic reactions.
Anti-IgE antibodies block the IgE-Fc receptor interaction and prevent subsequent allergic symptoms. Studies have demonstrated that camelid antibodies (HCAbs) have the potential to block IgE-FcεRI interaction and histamine release by basophils, thereby suppressing the triggers of allergy. The isolated single-chain antibodies are being tested in various studies for their efficacy in preventing specific immunoglobulin E antibodies mediated allergies.
The ability of camelid antibodies to block IgE-FcεRI interaction and histamine release by basophils was tested by Khaled et al. As per the study, which was published in Allergy, Asthma & Immunology Research, camel conventional antibodies (IgG1) had blocking activities of 43.9%, whereas camelid HCAbs (IgG2, and IgG3) had blocking rates of 72% and 96.6%. Additionally, the study demonstrated that, both IgG2 and IgG3 inhibited histamine release by 93.98% and 97.05%, compared with conventional antibody inhibition rate of 60.05%.
Researchers are trying to synthesize the single-chain antibodies as recombinant proteins in the laboratory and test them for their protective action as a stop sign to allergies. The inhibitory property, if proved successful in future studies, might facilitate strategies for the development of therapeutics for allergic diseases. Camelid antibodies will have to be evaluated for safety in humans, including their likelihood of inducing immune responses to foreign camel proteins.
Sources
- Fernandes, C., et al. (2017). Camelid Single-Domain Antibodies As an Alternative to Overcome Challenges Related to the Prevention, Detection, and Control of Neglected Tropical Diseases. Frontiers in immunology, 8, 653. doi:10.3389/fimmu.2017.00653
- Arbabi-Ghahroudi M. (2017). Camelid Single-Domain Antibodies: Historical Perspective and Future Outlook. Frontiers in immunology, 8, 1589. doi:10.3389/fimmu.2017.01589
- Khaled, A. Q., et al. (2015). Blocking of Histamine Release and IgE Binding to FcεRI on Human Basophils by Antibodies Produced in Camels. Allergy, asthma & immunology research, 7(6), 583–589. doi:10.4168/aair.2015.7.6.583
Further Reading
Last Updated: Sep 18, 2019