Research shows that depression is linked to the presence of inflammatory cytokines, in both healthy and ill patients.
Evidence for the Role of Cytokines in Depression
Cytokines play a role in brain development and function, and affect the neural circuits and transmitters within the brain, causing changes in the behavior. Evidence is accumulating that the levels of pro-inflammatory cytokines such as interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-alpha, and C-reactive protein (CRP), are linked to idiopathic major depression.
Macrophage releasing cytokines. Image Credit: Sciencepics / Shutterstock
Again, when inflammatory cytokines are administered, or when the innate immune system is stimulated, the resulting responses aim to restore normal function by controlling infection or healing injury. With chronic stimulation, however, or if the neurotransmitter pattern in the brain shows persistent alterations, the response may be psychiatric aberrations and depression.
Patients with depression not only show a higher level of inflammatory chemicals at rest but these levels are more sensitive than usual to stressful stimuli. IL-6 concentrations are increased and the anti-inflammatory effects of the glucocorticoid hormones following stress exposure are less pronounced.
Conversely, individuals who have more marked inflammatory responses to chronic illness are more prone to develop both fatigue and depression than the general population. This includes cancers, autoimmune disorders, and HIV infection which are linked to higher cytokine levels. This suggests the link between innate immune system activation and higher rates of depression in these individuals.
Thus inflammatory illnesses are more likely in individuals with high inflammatory cytokine levels in circulation, with greater risk of sickness and death due to these comorbidities than others.
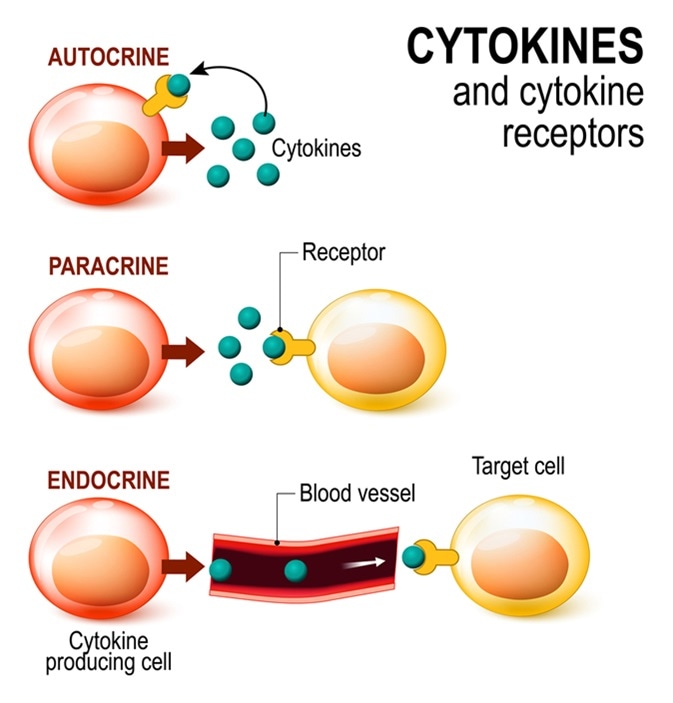
Cytokines and Cytokine receptor. signal transduction between cells. endocrine, paracrine and autocrine secretion. Image Credit: Designua / Shutterstock
How Cytokines Produce Effects on Behavior
High levels of inflammatory cytokines activate some neurotransmitter pathways which reduce growth factor concentrations while producing changes in monoamines, glutamate, and other peptide transmitters. The cytokines themselves may mirror or cause environmental factors of psychiatric stress such as trauma during childhood life, stress, lack of sleep and so on, as well as being the mechanism through which genetics expresses itself as incipient depression.
Chronic stress acts via physical signals such as danger-associated molecular patterns (DAMPs) to increase cytokine-mediated messages that eventually lead to depression and other medical diseases. Gut bacterial alterations also produce a direct change in the behavior and growth factor levels within the brain which are not linked to circulating cytokine changes, showing the possibility of a direct link between the gut and the brain, the gut-brain axis.
Cytokines can enter the brain via several pathways and recruit both endothelial and immune cells to produce more local cytokines, amplifying the effect on the brain. The depressive effect of these chemicals may be mediated by specific functional genetic polymorphisms such as one which codes for a serotonin reuptake promoter. This is supported by the anti-depressive effect of serotonin reuptake inhibitors in cytokine-induced depression.
Another interesting finding is that depressed patients without medical illness who do not respond to antidepressants have higher circulating inflammatory cytokine levels and CRP levels compared to responders. These patients may have traumatic childhood experiences which are later reflected into chronic inflammation and depression.
Therapeutic Implications
The importance of this knowledge is in the ability to work on developing drugs which prevent such signaling via inflammatory cytokines, as well as using other cytokines to prevent or to treat such behavioral effects. This is especially so when the patient already has known risk factors for cytokine-related depression, such as medical disease, psychosocial disturbances, or insomnia-related complaints. The administration of alpha-interferon, for instance, has been established to be associated with the development of iatrogenic depression in up to half of treated patients. This could be counteracted by more targeted interventions which alter the inflammatory response to produce clinical relief without intolerable side effects or threats to life.
Further Reading
Last Updated: Sep 3, 2018