Lissencephaly represents a developmental disorder resulting from abnormal neuronal migration. A wide spectrum of cerebral pathology can be seen in lissencephaly – from pachygyria and agyria (reduction and absence of cerebral convolutions, respectively) to subcortical band heterotopia where cerebral convolutions appear normal.
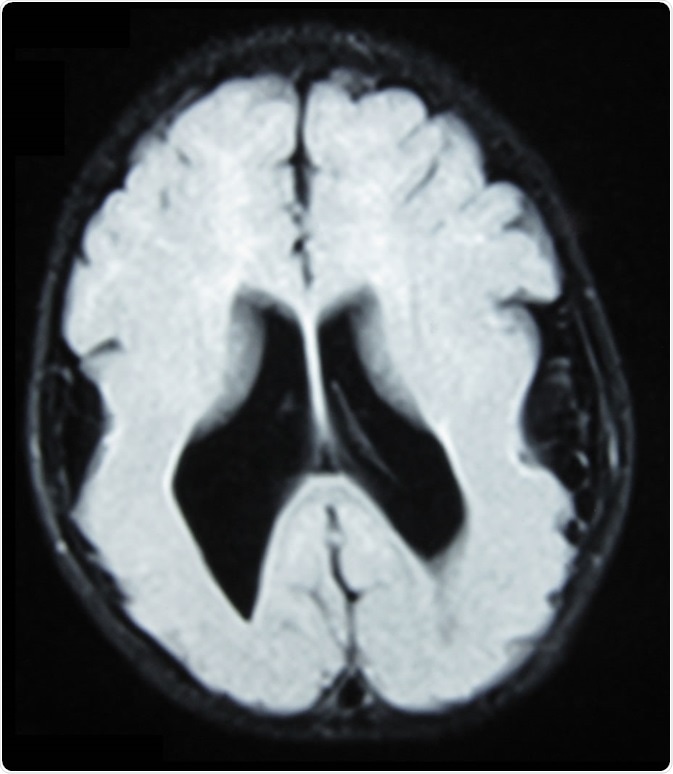
Brain MRI, T1 weighted, transverse plane, that shows lissencephaly, manifested as scarce and wide circumvolutions, mostly in the occipital, parietal, and temporal lobes. As aggregated findings, there is ventriculomegaly, no true Sylvian cissure, too thick gray matter and ectopic gray matter in the white matter. Image Credit: Ralphelg, https://creativecommons.org/licenses/by-sa/3.0/deed.en
Management of the disease
In lissencephaly, the brain's severe underlying malformations are not amenable to treatment; thus, supportive care is appropriate to ensure comfort and nursing requirements. Such a symptomatic approach depends on the severity and locations of the brain malformations.
Although medical management of seizures is available, it must be noted that epilepsy associated with lissencephaly and accompanying seizures are usually resistant to conventional therapy. Adrenocorticotropic hormones are a viable but not completely effective option in the treatment of infantile spasms.
For children with serious feeding difficulties, a gastrostomy feeding tube placement is often considered to preserve adequate nutrition. Ventriculoperitoneal shunts and encephalocele repair is pursued in certain cases of Walker-Warburg syndrome.
Prognosis
The outlook for all patients with lissencephaly is not good, especially for those with identifiable syndromes. Children with isolated lissencephaly may sit or roll, and rare patients with mild agyria and pachygyria will walk. Nevertheless, a majority of patients show no significant development beyond the 5-month level.
Even though most patients die before the age of two (regardless of the type of lissencephaly), survival to late childhood with near-normal intelligence is occasionally observed. As mentioned, certain mutations may result in milder cortical changes that are compatible with a substantially better prognosis.
Potential novel therapeutic strategies
Deeper insights into genes involved in lissencephaly and their mechanism of action may lead to novel therapies, and the experiments on rats and mice look promising. For example, by reexpressing the DCX gene after birth, aberrantly positioned neurons are stimulated to restart migration and restore neuronal patterning.
Furthermore, calpain-dependent proteolysis inhibitors can protect LIS1 from degrading, which results in the upsurge of LIS1 levels in mouse embryonic fibroblast cells and dorsal root ganglia neurons. This can overturn cytoplasmic dynein's aberrant distribution and other intracellular components responsible for lissencephaly and other similar disorders.
A long-term goal of this kind of research is to think of ways to translate the findings into human patients, which is not easy. It is hard to predict when such a breakthrough will occur; in the meantime, further understanding of genes and mechanisms is a step in the right direction.
Genomic insights into human cortical development, lissencephaly, and zika microcephaly - Kriegstein
Sources
- http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2967611/
- http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3565221/
- http://hmg.oxfordjournals.org/content/12/suppl_1/R89.long
- http://www.e-mjm.org/1996/v51n3/Lissencephalic_Syndromes.pdf
- Mohnish S, Young ID. Genetics for Pediatricians. Remedica London, 2005; pp. 45-51.
- Noggle CA, Horton AM, editors. The Encyclopedia of Neuropsychological Disorders. Springer Publishing Company, 2011; pp. 429-432.
Further Reading
Last Updated: Mar 1, 2021