Biophotonics is defined as the study of biological cells, tissues, and particles with the use of light in visible and near-visible range. It plays a major role in improving the imaging and therapeutic procedures that are commonly set for clinical purposes, including advances in lasers, optics, and fluorescence detectors. There are several primary techniques used in the biophotonic study.
Coherent anti-Stokes Raman scattering
Coherent anti-Stokes Raman scattering (CARS) is an optical method of mixing multiphotons for spectroscopy and distinct chemical microscopy with no accumulation of energy within the molecule. It is a noninvasive, non-destructive, and label-free method that provides vibrational imaging with high sensitivity, high velocity, and 3-D spatial resolution.
It has the advantage of reduced photodamage due to negligible energy transfer. It offers intrinsic 3-D segmentation and video imaging ability because of its nonsequential nature, which is of supreme importance in the field of histology and cell biology. Another advantage of CARS is the absence of fluorescence interruption.
It is used in imaging of living cells and ex vivo tissues (e.g., DNA, lipids, and proteins) with varied vibrational contrasts.
Oblique plane microscopy
Oblique plane microscopy (OPM) is a light sheet microscopic method that involves a single, high numerical aperture microscope for illuminating an oblique plane of the sample and collection of fluorescence from the illuminated plane. The correction optics is placed between the main microscope and the charge-coupled camera device, to obtain the image of the tilted plane on the specimen.
OPM technique can be applied with a conventional microscope to image specimens mounted on coverslips and tissue culture dishes or plates with very low photobleaching and phototoxicity. This can also be operated at high velocity as the obtained image is optically sectioned in the absence of moving parts.
Fluorescence lifetime imaging microscopy
Fluorescence lifetime imaging (FLIM) is a method that works based on the principle of fluorescence lifetime (time required by a fluorophore to return to its ground state from excited state). FLIM is used to represent the spatial dispersion of molecules’ lifetime in excited state within microscopic images. FLIM system is executed in two domains: frequency and time.
The fluorescence lifetime information is used in local environment sensing, detection of molecular interactions, detection of conformational changes, discrimination of multiple labels or background removal, tissue characterization by auto fluorescence and characterization, and quality control of new materials.
Confocal microscopy
Confocal microscopy is a conventional optical imaging method that has improved lateral and axial resolution and capability of distinct imaging compared to FLIM microscopy. This is achieved by elimination of the scattered fluorescence by introducing a pinhole or a screen combination in this method. The focus of the objective lens is made conjugate with the pinhole and, therefore, it is termed as confocal pinhole.
Confocal microscopy is used in the high-degree imaging of dense biological samples (in vivo tissues, without excision, fixation, and partitioning of tissues).
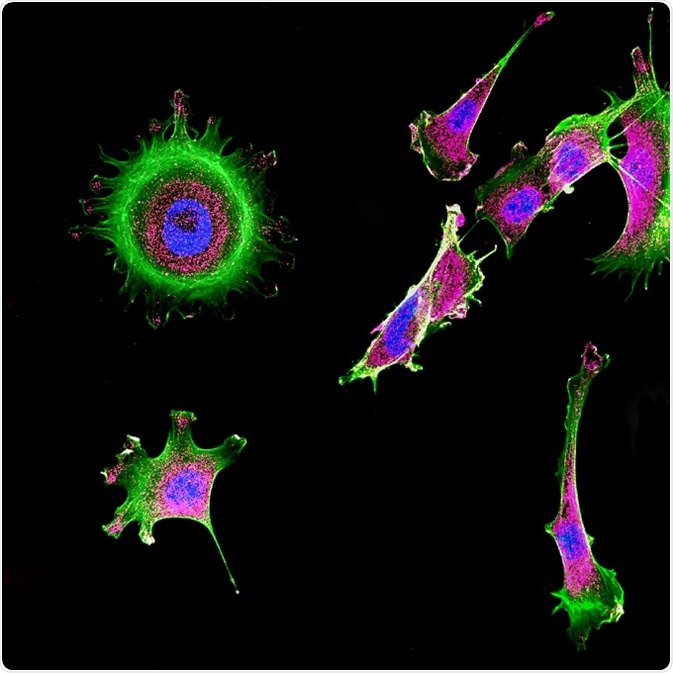
Immunofluorescence of multiple tumor cells grown in tissue culture for cancer research and visualized via confocal microscopy. © DrimaFilm/Shutterstock.com
Multiphoton microscopy
Multiphoton microscopy (MPM) is a nonlinear imaging technique used to obtain micron-level accurate information from unprocessed tissue through emissions produced by energized intrinsic tissues. The working principle of MPM is based on the instantaneous absorption of two incident photons from a pulsed infrared laser source. In this method, excitation is limited to a tiny focus resulting in prior optical segmentation without the necessity of confocal aperture.
MPM is used as a substitute for confocal microscopy due to its advantages of deep penetrated imaging, with reduced photodamage and photobleaching and is considered to develop the in vivo imaging process.
Targeted molecular imaging
The technique of target molecular imaging involves analyzing micron-level biological processes by using in vivo methods. The process includes the use of target contrast agents (substances that improve the internal visibility of the biological structures) that are detected using optical imaging modalities and identification of targets on cells or tissues. This method is used to analyze the shape and role of the molecular system by generating the signals incident from the molecules. Therefore, the produced image describes the 3-D spatial distribution of the targeted molecules in the tissue, specifies the diagnostic data at the molecular level, and shows the functional cell properties.
The technique is used in oncology studies for the diagnostics of malignancies, discovery of metastatic disease, detection of tumors, determining treatment targets, and estimating effectiveness of the treatment.
Optical projection tomography
Optical projection tomography (OPT) is a method developed for 3-D imaging of tiny biological specimens in the mesoscopic range. Here, the specimens are appended in refractive index matching liquids and rotated to an angular series. Then, images are taken for each angular position by a CCD camera and the recalculated as 3-D image of the sample by computer software.
OPT has the advantage of detecting fluorescent and colored dyes that are established for gene or tissue-specific staining for imaging of 3-D shapes of organs and tissues such as restoration of vertebrate embryos and observation of 3-D anatomy of organs.
Further Reading
Last Updated: Jul 19, 2023