Jun 13 2014
Fully Automated Instrument Delivers Fast, Efficient Results
Beckman Coulter Life Sciences is set to transform the use of flow cytometry in the routine clinical laboratory with its latest offering, the Aquios CL Clinical Flow Cytometer*. Fully automated, with a small footprint, the instrument is the first authentic ‘LOAD & GO’ cytometry system.
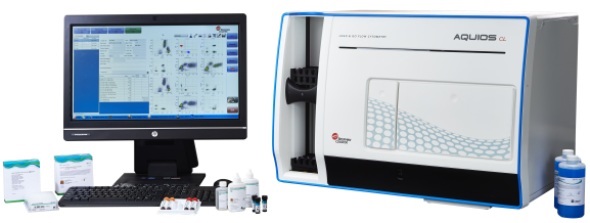
Routine clinical tests can be run by lab technologists using the new Aquios CL flow cytometer from Beckman Coulter Life Sciences.
The Aquios CL is designed specifically to streamline workflow and reduce backlogs when handling routine applications, such as immunophenotyping. The Aquios CL delivers first results within 20 minutes, with subsequent results at a rate of 25 samples per hour for up to a full 96-well plate.
As easy to learn and use as a haematology analyser, it can be operated with minimal training, freeing up resources for more complex assay analysis. By removing manual processes, the high level of automation helps prevent sample processing errors. Its cap-piercing technology reduces staff exposure to biohazardous samples.
This fully integrated system includes a 40-tube capacity autoloader able to handle a variety of tube sizes, with continuous, random loading and unloading. There is a separate single-tube loader for STAT samples. Sample preparation, reagent monitoring, barcode scanning, data analysis and LIS connectivity are all automated.
“The Aquios CL helps deliver operational savings and improve workflow in the routine lab,” said Mario Koksch, vice president and general manager of the Cytometry Business Unit for Beckman Coulter Life Sciences. “This ‘LOAD & GO’, fully automated system performs fast and efficient testing for routine applications such as immunophenotyping, freeing up experienced staff for more complex analysis.”
Intuitive software makes the system fast and easy to use. ‘Smart Track Reagent Monitoring’ tracks reagent use and test progress, with barcode tracking eliminating manual quality control and reagent logs. If the QC fails, the system automatically alerts by text message or email for immediate intervention.
The first tests available for the Aquios CL are Tetra-1 (CD45/4/8/3), Tetra-2+ (CD45/56+16/19/3), and Tetra Combo (both Tetra-1 and -2+) for immune status monitoring.
* Pending submission and clearance by the United States Food and Drug Administration; not yet available for in vitro diagnostic use. The device has not yet been licensed in accordance with Canadian law. The performance specifications of this product have not been established. Les spécifications de rendement de l'instrument n'ont pas été établie.
Beckman Coulter, AQUIOS, Load & Go, and the stylized logo are trademarks of Beckman Coulter, Inc. and are registered with the USPTO.
Source: https://www.beckmancoulter.com/