Sponsored Content by MaxCyte, Inc.Reviewed by Maria OsipovaApr 13 2023
Despite progress in human genetic medicine, individuals suffering from rare disorders, such as Duchenne muscular dystrophy (DMD), continue to face shortened life expectancies with a limited range of treatment options.
CRISPR-Cas9 technology has enabled scientists to accurately target specific genomic sequences, enabling precise alterations to disease-causing genetic mutations.
In vivo editing requires efficient, direct delivery of functional CRISPR components to the patient’s body; research on mice has indicated that adeno-associated viruses can successfully deliver CRISPR-Cas9 in vivo.
However, there is a concern that prolonged expression of CRISPR components may result in adverse effects, such as non-specific cleavage of crucial genes involved in cellular function or unintended mutations in oncogenes that could potentially lead to tumor formation.
In addition, the effectiveness of AAV for delivery may be diminished due to immunological responses. For patients to reap the full benefits of advances in gene editing, a transient delivery system is needed to enable CRISPR-Cas9 to target a specific DNA sequence, make the therapeutic edits, and then degrade, minimizing the chance of undesirable edits.
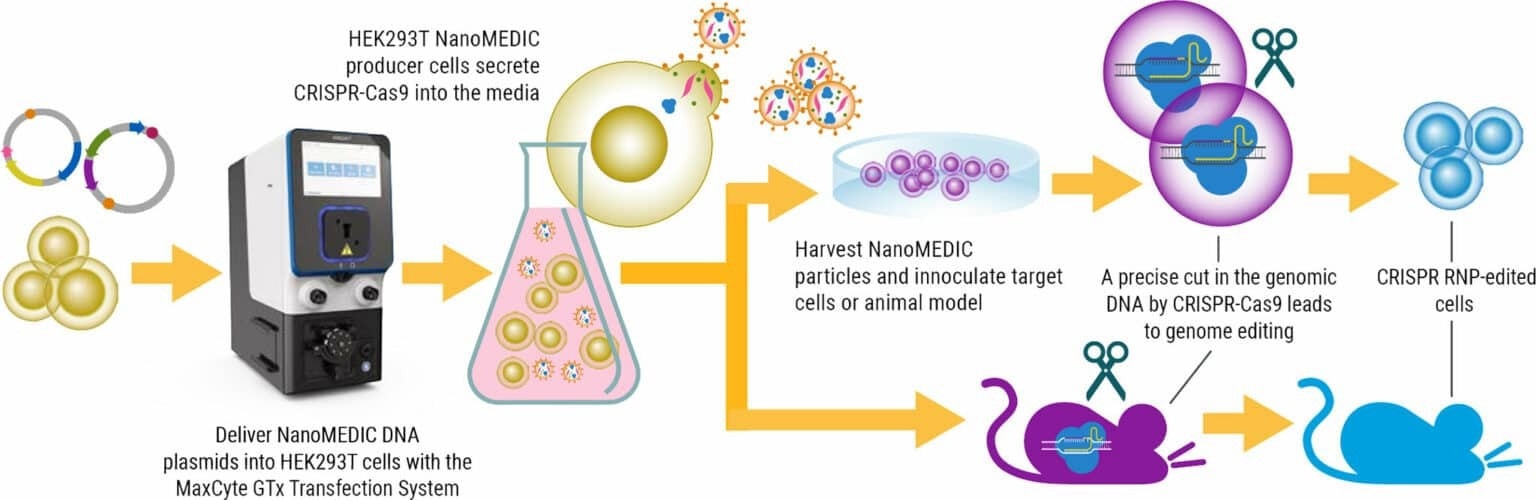
Image Credit: MaxCyte, Inc.
Rationale
A recent study in Nature Communications introduces a new, temporary delivery system known as NanoMEDIC. These engineered extracellular nanovesicles contain a CRISPR-Cas9 ribonucleoprotein (RNP) complex and retain the delivery properties of lentivirus without the long-term risks associated with viral vector integration.
NanoMEDIC effectively induces genome editing in several cell types, including muscle myoblasts, T cells, human induced pluripotent stem cells (iPSCs) and monocytes. NanoMEDIC was also demonstrated to be effective in vivo in immunodeficient mice.
Having demonstrated the feasibility of producing NanoMEDIC at a small scale, the researchers aimed to develop a scalable method for production using a cGMP-compatible Flow Electroporation® system that could be utilized for industrial manufacturing purposes.
Technical approach
HEK293T suspension culture was utilized, eliminating animal components and enhancing cell handling via higher-density cell culture. Electroporation conditions were optimized to increase NanoMEDIC yields. Process refinements included fine tuning the amount of the VSV-G envelope plasmid in the transfection mixture, post-electroporation DNase treatment of transfected cells and the use of a custom, high-energy electroporation protocol.
Notably, although electroporation conditions were initially determined using 60 x 106 cells, MaxCyte’s scalable Flow Electroporation® enabled the seamless scaling up of NanoMEDIC production to 1.2 x 109 cells.
Quantification of active CRISPR-Cas9 RNP complex in an in vitro enzymatic assay confirmed that yields between small-scale and large-scale NanoMEDIC production were comparable.
Conclusion
The findings of this study describe a transient CRISPR-Cas9 delivery system with potential not only for gene therapy but also for delivering other cargo, including viral antigens with possible applications for vaccine development.
The NanoMEDIC manufacturing process is free from animal-derived components and employs MaxCyte Flow Electroporation® to facilitate scalable production of NanoMEDIC from up to billions of cells.
References and further reading
Gee P, Lung MSY, Okuzaki Y, et al. Extracellular nanovesicles for packaging of CRISPR-Cas9 protein and sgRNA to induce therapeutic exon skipping. Nat Commun. 2020;11(1):1334. Published 2020 Mar 13. doi:10.1038/s41467-020-14957-y
About MaxCyte, Inc.
MaxCyte is a leading commercial cell-engineering company focused on providing enabling platform technologies to advance innovative cell-based research as well as next-generation cell therapeutic discovery, development and commercialization. Over the past 20 years, we have developed and commercialized our proprietary Flow Electroporation® platform, which facilitates complex engineering of a wide variety of cells.
Our ExPERT™ platform, which is based on our Flow Electroporation technology, has been designed to support the rapidly expanding cell therapy market and can be utilized across the continuum of the high-growth cell therapy sector, from discovery and development through commercialization of next-generation, cell-based medicines. The ExPERT family of products includes: four instruments, the ATx™, STx™, GTx™, and VLx™; a portfolio of proprietary related processing assemblies or disposables; and software protocols, all supported by a robust worldwide intellectual property portfolio.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.