A recent study published in the journal Nature Aging reports that small extracellular vesicles (sEVs) in plasma from young mice counteract preexisting aging.
Study: Small extracellular vesicles from young plasma reverse age-related functional declines by improving mitochondrial energy metabolism. Image Credit: Ground Picture / Shutterstock.com
Can aging be reversed?
Recent studies have demonstrated that blood from young mice can rejuvenate the brain, liver, bone, skeletal muscle, pancreas, and heart of aged mice by connecting their circulatory systems through heterochronic parabiosis. Likewise, plasma infusions can recapitulate the phenotypes conferred by blood exchange in heterochronic parabiosis.
Intensive efforts to identify the plasma factors implicated in reversing age-related impairments have led to the discovery of rejuvenation, pro-youth, or anti-aging factors; however, their mechanisms of action remain unclear.
About the study
EVs are nano-sized membranous vesicles that circulate throughout the bloodstream and serve as intercellular messengers by exchanging cargoes between cells. In the current study, researchers explored the rejuvenating effects of sEVs.
Researchers initially purified sEVs from young and aged male mice that were two and 20 months old, respectively. Young plasma sEVs were then evaluated for their ability to potentially increase the lifespan of old mice. Aged mice received an equal volume of intravenous young plasma sEVs or phosphate-buffered saline (PBS) once weekly until death.
Study findings
Young sEV administration significantly increased the frailty index score and median lifespan of aged mice by 12.4%. Furthermore, the estimated biological age of sEV-treated mice was 15.1 months, compared to their chronological age of 24 months.
Treatment with young sEVs improved testosterone levels, sperm counts, motility, and sperm chromatin integrity, as well as decreased sperm DNA fragmentation. Fertile female mice were mated with young or aged mice, followed by the visual examination of embryo implantation 4.5 days later.
Mating with young males resulted in 7.7 implantation sites/pregnant females, whereas aged mice failed to establish implantation sites. Nevertheless, treatment of aged mice with young sEVs significantly improved fertility defects, with 3.4 sites/pregnant female.
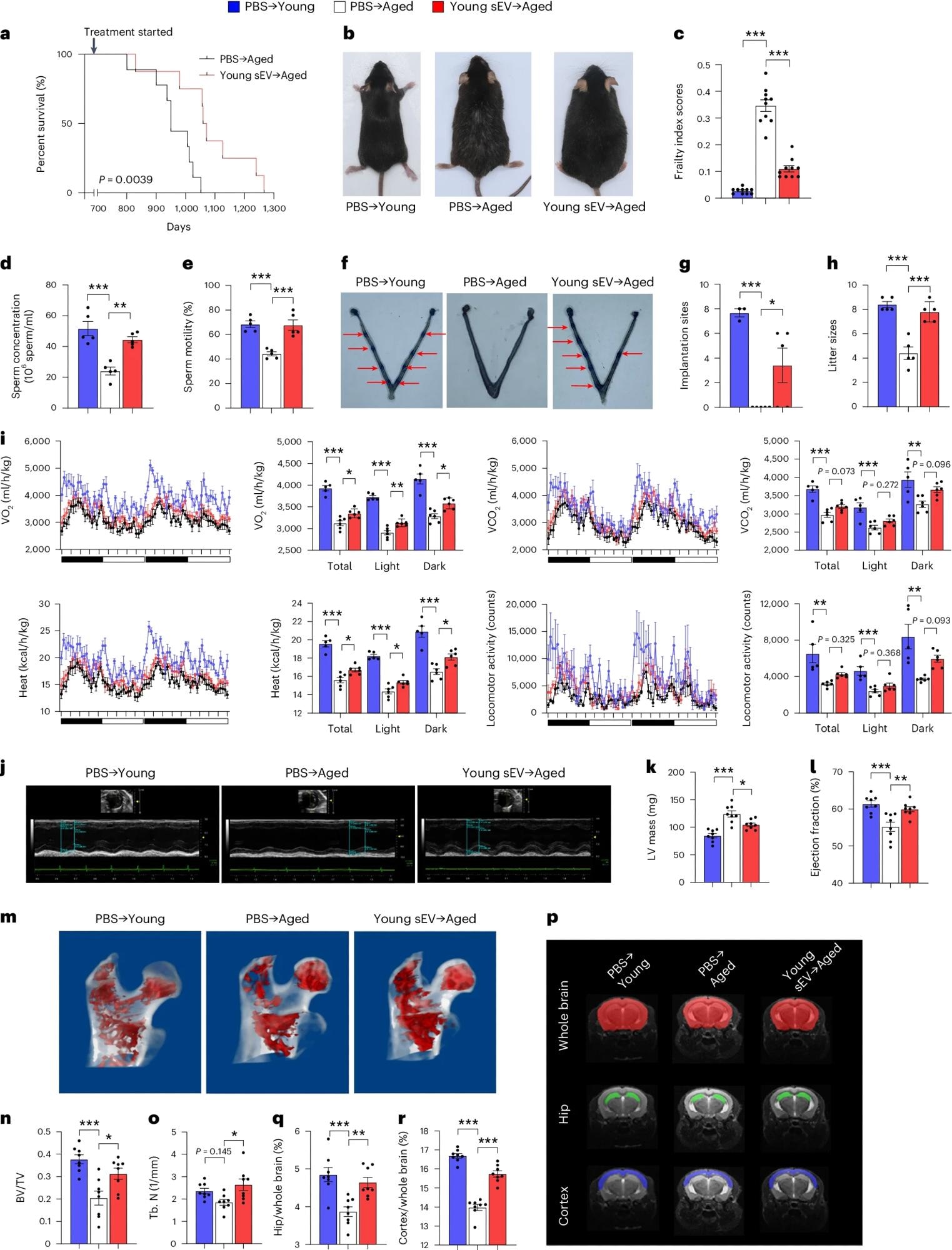 Aged male mice (20 months) were intravenously injected with 200 μl of PBS or young sEVs (2 months) once a week, and they were monitored to determine either the survival time or whole-body physiology. Young male mice (2 months) were simultaneously injected with PBS to serve as a control group. a, Kaplan–Meier survival curves in each group (n = 8–9). b, Representative images of mice in each group after 7 months of treatment. c, Mean frailty index scores in each group after 4 months of treatment (n = 10). d,e, Sperm counts and motility in each group (n = 5). f,g, The number of implantation sites visible as blue bands in the uterus of female mice that were mated with the male mice from each group. Representative images (red arrows indicate implantation sites) and quantitative data (n = 3 for PBS → Young; n = 5 for else) are shown. h, The number of offspring sired by the male mice in each group (n = 5). i, Indirect calorimetry measurements of O2 consumption, CO2 release, heat production and locomotor activity in each group (n = 5 for PBS → Young; n = 6 for else). j–l, Echocardiographic measurements of cardiac dimensions and indices of cardiac function in each group. Representative M-mode echocardiographs and quantitative values of LV mass and EF (n = 8) are shown. m–o, Micro-CT analysis of the trabecular microarchitecture of the proximal femur in each group. Representative 3D images of the proximal femur and quantitative values of BV/TV and Tb.N (n = 8) are shown. p–r, MRI-based morphometric analyses of the hippocampus and cortex in each group. A representative MRI scan of a slice is shown, and the volume ratios (hippocampus/whole brain and cortex/whole brain) were calculated (n = 8). Significance was determined using the log-rank test in a and using one-way ANOVA followed by Dunnett’s multiple comparison test in c–e, g–i, k, l, n, o, q and r. *P < 0.05, **P < 0.01 and ***P < 0.005.
Aged male mice (20 months) were intravenously injected with 200 μl of PBS or young sEVs (2 months) once a week, and they were monitored to determine either the survival time or whole-body physiology. Young male mice (2 months) were simultaneously injected with PBS to serve as a control group. a, Kaplan–Meier survival curves in each group (n = 8–9). b, Representative images of mice in each group after 7 months of treatment. c, Mean frailty index scores in each group after 4 months of treatment (n = 10). d,e, Sperm counts and motility in each group (n = 5). f,g, The number of implantation sites visible as blue bands in the uterus of female mice that were mated with the male mice from each group. Representative images (red arrows indicate implantation sites) and quantitative data (n = 3 for PBS → Young; n = 5 for else) are shown. h, The number of offspring sired by the male mice in each group (n = 5). i, Indirect calorimetry measurements of O2 consumption, CO2 release, heat production and locomotor activity in each group (n = 5 for PBS → Young; n = 6 for else). j–l, Echocardiographic measurements of cardiac dimensions and indices of cardiac function in each group. Representative M-mode echocardiographs and quantitative values of LV mass and EF (n = 8) are shown. m–o, Micro-CT analysis of the trabecular microarchitecture of the proximal femur in each group. Representative 3D images of the proximal femur and quantitative values of BV/TV and Tb.N (n = 8) are shown. p–r, MRI-based morphometric analyses of the hippocampus and cortex in each group. A representative MRI scan of a slice is shown, and the volume ratios (hippocampus/whole brain and cortex/whole brain) were calculated (n = 8). Significance was determined using the log-rank test in a and using one-way ANOVA followed by Dunnett’s multiple comparison test in c–e, g–i, k, l, n, o, q and r. *P < 0.05, **P < 0.01 and ***P < 0.005.
Following continuous mating for one month, young and aged males produced eight and four pups/litter, respectively. Furthermore, treating aged mice with young sEVs produced nearly as many pups as young males.
Aged mice treated with young sEVs also exhibited higher oxygen consumption and carbon dioxide production than PBS recipients, thus suggesting partial restoration of metabolic health to the levels of young mice. Treatment also significantly improved echocardiography parameters and bone architecture in aged mice. Magnetic resonance imaging (MRI) showed cortical atrophy in aged mice, which was mitigated upon treatment with young sEVs.
Aged mice exhibited increased senescence-associated β-galactosidase (SA-β-gal) activity in the spleen, kidney, liver, lung, testis, and hippocampus compared to young mice. However, treating aged mice with young sEVs, even for two weeks, led to a rapid reduction in SA-β-gal levels in these organs.
Young sEV treatment also restored intracellular reactive oxygen species (ROS) levels in aged mice to those observed in young mice and eliminated the accumulation of excess advanced glycation end products. Proteomic analyses indicated that young sEVs might reverse age-associated degenerative changes and exert rejuvenating effects.
The researchers also evaluated the effects of treatment with sEVs isolated from young humans on age-related impairments in mice. To this end, treating aged mice with sEVs from young humans effectively ameliorated their cognitive deficits, enhanced endurance capacity, and supported the recovery of mitochondrial activity in the muscle.
While sEVs efficiently transport nucleic acids, proteins, and lipids, most studies have focused on the ribonucleic acid (RNA) component of sEVs, particularly microRNAs (miRNAs). To elucidate the role of certain miRNAs in plasma sEVs, the differential expression of miRNAs was assessed in plasma between young and aged mice through small RNA deep sequencing.
Additional experiments identified representative miRNA cargoes of young and aged sEVs. More specifically, miR-455-3p, miR-144-3p, and miR-149-5p represented the young state, whereas miR-34a-5p, miR-29a-3p, and miR-29c-3p represented the aged state. Notably, peroxisome proliferator-activated receptor γ coactivator 1 α (PGC-1α) was a common target negatively regulated by miR-34a-5p, miR-29a-3p, and miR-29c-3p.
Furthermore, miR-455-3p, miR-144-3p, and miR-149-5p could be indirect stimulators of PGC-1α, as their downstream target genes show an inverse correlation with PGC-1α. Aged mice exhibited significantly reduced levels of PGC-1α expression in the muscle and hippocampus compared to young mice, and young sEV treatment of aged mice increased its expression.
Silencing PGC-1α using a small-interfering RNA (siRNA) significantly diminished the benefits observed with young sEV treatment on mitochondrial respiration. Additional investigations confirmed that miR-455-3p, miR-144-3p, and miR-149-5p in young sEVs were rejuvenating miRNAs, whereas miR-34a-5p, miR-29a-3p, and miR-29c-3p in aged sEVs were pro-aging miRNAs.
Conclusions
Repeated administration of young sEVs enhanced the physiologic function and physical performance of aged mice. In the short term, young sEV treatment rapidly improved the whole body. In contrast, young sEV treatment revitalized the performance and properties of aged tissues to levels observed in young mice over longer periods.
Taken together, the study findings indicate that young sEVs could lead to promising opportunities to rejuvenate aging tissues and improve well-being and lifespan.
Journal reference:
- Chen, X., Luo, Y., Zhu, Q., et al. (2024). Small extracellular vesicles from young plasma reverse age-related functional declines by improving mitochondrial energy metabolism. Nature Aging. doi:10.1038/s43587-024-00612-4