Cystic fibrosis is a hereditary disease caused by a mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. The CFTR protein is an ion channel that carries salts into and out of cells. When CFTR is defective, water and salt are out of balance in the cells, and a thick mucus builds up in the tissue, leading to problems in many organs, especially the lungs. Mucus blocks the airways and promotes lung infection. Cystic fibrosis shortens the lifespan of the sufferers to an average of 28 years.
There are some new approved therapies on the market for cystic fibrosis that are designed to treat many of the specific mutations of CFTR that cause cystic fibrosis. As well, auxiliary therapies such as improved antibiotic coverage are available to help resolve the complications of cystic fibrosis such as lung infections. However, there is still no cure for the disease.
Gene editing using the CRISPR/Cas9 system is a new technology that could potentially lead to a cure for cystic fibrosis.
CF Foundation | How Gene Editing Could Be Used for CF
What is CRISPR?
CRISPR stands for Clustered Regularly Interspaced Short Palindromic Repeats in reference to the repeating DNA sequences found in the genomes of bacteria. They function as a part of the immune system of the organism. When attacked by a virus, the CRISPR system destroys the genome of the viral invader and incorporates bits of the viral genome into its sequence. These pieces of viral DNA are known as spacers, and they allow the CRISPR system to quickly detect genetic material from the same virus in the future.
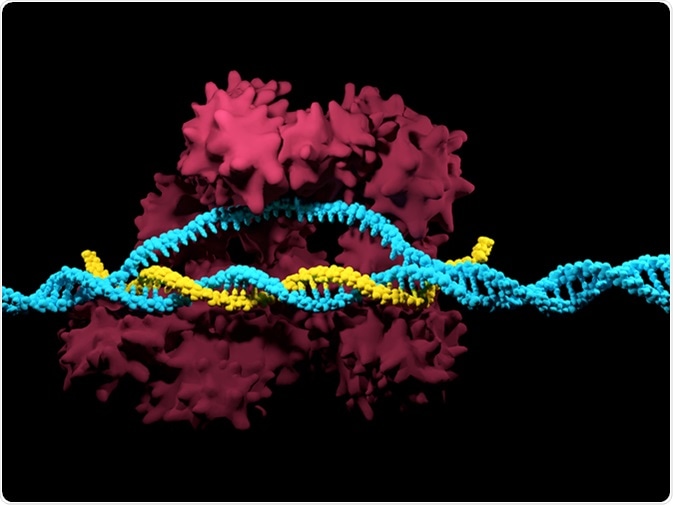
CRISPR-Cas9 Illustration. Image Credit: Meletios / Shutterstock
The bacterial CRISPR system has been adapted by researchers to modify just about any target DNA. Scientists synthesize short RNA sequences called ‘guide RNA’ to direct the CRISPR machinery to the DNA target. It can then silence a gene by cutting the strand, introducing a gap, or it can actually change the sequence using a repair template.
CRISPR and Cystic Fibrosis
CRISPR has been a revolutionary new technology in the field of molecular biology, and its implications for medical science were immediately recognized when the first studies were published in 2013 showing how the CRISPR system could be used for genomic engineering. The field has progressed very rapidly since then. Scientists have been investigating CRISPR for a number of human diseases, including cancer, HIV, and cystic fibrosis.
A 2013 study in Cell Stem Cell demonstrated restoration of CFTR functionality using CRISPR. The researchers cultured intestinal stem cells from cystic fibrosis patients and corrected the mutation at the CFTR locus resulting in expression of the correct gene and full function of the protein. That research established the potential utility of the application of CRISPR for cystic fibrosis.
To pursue the potential of gene editing, Cystic Fibrosis Foundation Therapeutics Inc. awarded up to $5 million to the gene editing firm Editas Medicine Inc. to advance technologies that could be developed for use in cystic fibrosis. The goals of the collaboration include developing gene editing therapies to repair the CFTR gene, developing methods of delivering the editing components into cells, and testing potential therapies in animals.
Delivery Challenges
In addition to developing the fundamental therapeutic gene editing strategies, scientists must also figure out how to get the editing apparatus into the cells of the patient before it can be deployed as a treatment.
There are two established options for delivery of gene editing therapies for cystic fibrosis. One would be editing the lung cells in vivo, or inside the living patient. Another would be modification of cells outside the patient, and then delivery of the cells into the desired tissues.
However, other innovative methods are being investigated. Previously, circular segments of DNA, called plasmids, have been used to deliver functional CFTR genes to lung cells, and that method has been found to stabilize lung function in patients. A similar strategy using a plasmid to encode the gene-editing machinery could also be successful.
Other delivery strategies being investigated include viral vectors (such as adeno-associated virus and lentivirus) and nanocomplex formulations of lipids and peptides containing DNA. Some combinations of viral and non-viral delivery, where different components of the system are delivered by different means, may be successful, as well.
References
- CRISPR: A game-changing genetic engineering technique, http://sitn.hms.harvard.edu/flash/2014/crispr-a-game-changing-genetic-engineering-technique/
- Cystic Fibrosis News Today, CRISPR/Cas9 Approach for Cystic Fibrosis Treatment, https://cysticfibrosisnewstoday.com/crisprcas9-approach-for-cystic-fibrosis/
- Functional Repair of CFTR by CRISPR/Cas9 in Intestinal Stem Cell Organoids of Cystic Fibrosis Patients, http://www.cell.com/cell-stem-cell/fulltext/S1934-5909(13)00493-1?_returnURL=http%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS1934590913004931%3Fshowall%3Dtrue
- CFFT Makes First Major Award to Advance Gene Editing Research in CF, https://www.cff.org/
- Impact of gene editing on the study of cystic fibrosis, https://www.ncbi.nlm.nih.gov/pubmed/27325484
- Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: a randomised, double-blind, placebo-controlled, phase 2b trial, https://www.ncbi.nlm.nih.gov/pubmed/26149841
- Multiplex Genome Engineering Using CRISPR/Cas Systems, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3795411/
Further Reading
Last Updated: Dec 29, 2022