Ribavirin, the nucleoside analog 1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxamide, is a broad spectrum direct antiviral agent. This drug was discovered more than 40 years ago and is efficient both in vitro and in vivo against several RNA or DNA viruses.
Ribavirin, tribavirin, C8H12N4O5 molecule. Image Credit: Bacsica / Shutterstock
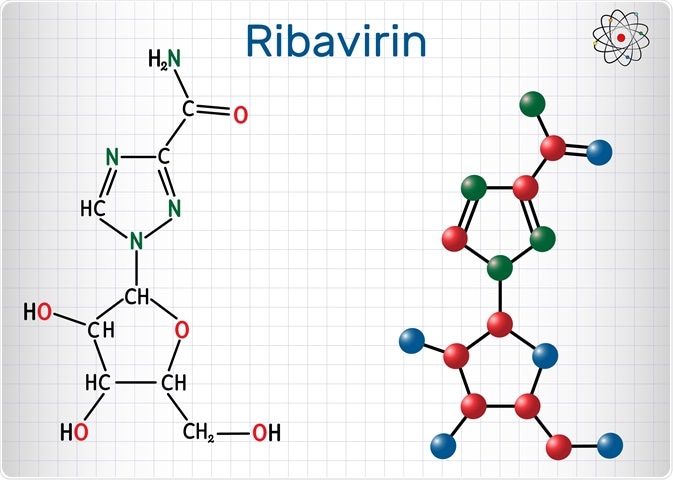
Structure and analogs
Ribavirin represents a water-soluble, guanosine nucleoside analog that mimics other purines, including inosine and adenosine. Structure-activity relationship studies reveal that the 1,2,4-triazole ring, carboxamide group, and beta-D-ribofuranosyl moiety are pivotal for this drug's antiviral activity.
Ribavirin's base moiety closely resembles the monocyclic base found in nicotinamide and 5-aminoimidazole-4-carboxyamide ribonucleoside are both naturally occurring metabolites. Furthermore, its sugar moiety (present as ribose with a hydroxy group at the 2' carbon position) allows ribavirin to be preferentially active in RNA-related metabolism.
Upon absorption, cellular enzymes convert the compound into several metabolites, including the monophosphates, diphosphates and triphosphates, as well as the deribosylated base. These derivatives consequently inhibit viral nucleic acid synthesis, principally by altering the normal formation of messenger RNA.
A plethora of analogs of ribavirin have been synthesized, most notably 3-carboxamidine derivative (found to be similar to ribavirin) and thiocarboxamide, active only against DNA viruses. Ribamidine has been shown as highly active against the Punta Toro virus in mice and the Pichinde virus in hamsters.
The nucleoside antibiotic mizoribine is structurally in close relationship with ribavirin. Mizoribine and its 5'-phosphate are active against L-1210 cells (mouse lymphocytic leukemia cell line). The mechanism of action of this substance involves the inhibition of inosine monophosphate dehydrogenase.
Production of ribavirin
Different techniques can be employed to produce ribavirin, and among the most commonly used are synthetic, enzymatic and fermentative methods. Chemical synthesis is the most frequently encountered in the current industry. However, because of the troublesome manipulation and the ecologically unfriendly process, this production method is far from ideal.
In the enzymatic method, purine nucleoside phosphorylase is used to catalyze ribavirin synthesis from its precursors – purine nucleoside and 2H-1,2,4-triazole-3-carboxamide (TCA). Nonetheless, the cost of this technique is high due to the high price of precursors and expensive enzyme sources.
The fermentative method was described in 1976 where TCA was added to the culture of nucleoside producing strains of bacterial genera Brevibacterium or Bacillus; ribavirin was subsequently synthesized from endogenous nucleoside catalyzed by the natural purine nucleoside phosphorylase. The main drawback is a low yield of ribavirin.
A combination of traditional fermentation with enzymatic synthesis process was successfully used to establish a novel ribavirin biosynthesis pathway. By overexpressing purine nucleoside phosphorylase, the previous end product purine nucleoside can be successfully converted to ribavirin. Guanosine-producing strain derivate was screened out as the optimal ribavirin-producing strain.
Ribavirin: Evidence-Based Health Information Related to COVID-19
References
Further Reading
Last Updated: Dec 22, 2022