Skip to
Energy transformations in metabolism
The energy for various functions of the human body comes from the nutrient molecules that have been broken down, in a process called metabolism. Metabolism comprises of two major parts: anabolism (building up, also called biosynthesis) and catabolism (breaking down).
The exact nature of catabolic reactions differs from organism to organism and organisms can be classified based on their sources of energy and carbon:
- in organotrophs, organic sources are used as a source of energy
- in lithotrophs, inorganic substrates are used
- in phototrophs, sunlight is used as chemical energy
- in heterotrophs, organic compounds are used as a source of energy (the compounds are not synthesized by the organism, but obtained through food).
The basic common reactions in catabolism include oxidation-reduction (redox) reactions that involve the transfer of electrons from reduced donor molecules, such as organic molecules, water, ammonia, hydrogen sulfide or ferrous ions, to acceptor molecules, such as oxygen, nitrate or sulfate.
In humans and animals (heterotrophs), redox reactions involve complex organic molecules being broken down to simpler molecules, such as carbon dioxide and water. In photosynthetic organisms such as plants and cyanobacteria (phototrophs), these electron-transfer reactions do not release energy. These reactions just help store energy absorbed from sunlight.
Energy from foods
In humans, the body’s energy comes from the fats, carbohydrates, and proteins in food, making us heterotrophs. Of the three organic molecules, fat is the most concentrated source of energy because it furnishes more than twice as much energy for a given weight as protein or carbohydrate.
Energy requirements are ordinarily expressed in terms of calories or kilocalories. A kilocalorie (kcal) is the amount of heat energy required to raise the temperature of one kilogram of water by one degree Celsius. Fat provides the most energy per mass, at 9 kcal/g, followed by proteins and carbohydrates (4 kcal/g) and hydrated carbohydrates (1.3 kcal/g). Lipids are broken down into fatty acids, proteins into amino acids, and carbohydrates into glucose. These products then undergo redox reactions.
The Basal Metabolic Rate (BMR) is the heat eliminated from the body at rest when temperature is normal. An average person requires 2,000-2,400 Calories per day, while a large man doing heavy work may require up to 6,000 Calories per day. Children’s energy needs vary widely based on their age, size, and activity level.
Metabolism
Catabolic processes
Overall, both processes of metabolism, catabolism and anabolism, must occur concurrently because catabolism provides the energy necessary for anabolism. The body utilizes energy for a variety of functions. Energy is needed to carry out mechanical work which involves the change in location or orientation of a body part or the cell itself. This includes muscle movement. In addition, there is molecular transport and synthesis of biomolecules.
ATP (adenosine triphosphate) is the energy molecule that transfers chemical energy in human cells. In general, the energy to synthesize ATP molecules must be obtained from food molecules. ATP is mainly synthesized in the mitochondria in the cells, with some additional ATP synthesized in the cytoplasm.
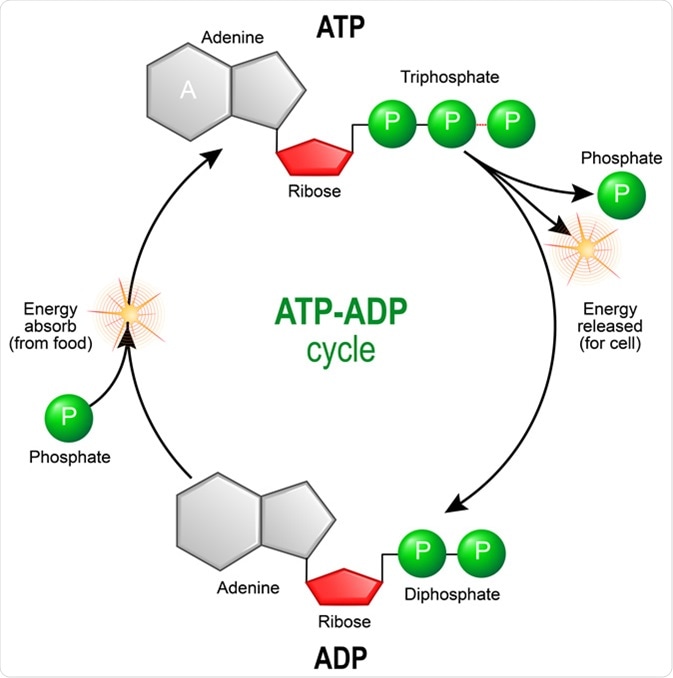
ATP ADP cycle. Adenosine triphosphate (ATP) is a organic chemical that provides energy for cell. intracellular energy transfer. Adenosine diphosphate (ADP) is organic compound for metabolism in cell. Illustration Credit: Designua / Shutterstock
Catabolism can be divided into three main stages:
- Digestion – large organic molecules (proteins, lipids, carbohydrates) are digested into their smaller components (fatty acids, amino acids, and glucose, respectively) outside the cell by digestive enzymes, such as glucoside hydrolases for carbohydrates and pepsin for proteins. This occurs in the digestive tract of humans.
- Energy release – the smaller components are moved into the cells by active transport proteins and converted to smaller molecules, typically acetyl coenzyme A (acetyl CoA), which releases some energy. In the cytoplasm, glucose is further transformed to pyruvate, which causes synthesis of two ATP molecules. In humans, these small molecules are transported across the digestive system tissues into circulatory tissues, then distributed throughout the body to where they are needed to produce energy.
- ATP production – in the citric acid cycle (also called the Kreb’s or TCA cycle), CoA’s acetyl group is oxidized to water and carbon dioxide. The energy released from this is stored in ATP by reduction of the coenzyme adenine dinucleotide (NAD+) into NADH in the electron transport chain. This process is called oxidative phosphorylation and releases carbon dioxide as a waste product.
The different organic molecules provide various amounts of ATP. Each molecule of fatty acid releases over 100 molecules of ATP, and each amino acid molecule releases nearly 40 ATP molecules.
Amino acids can be oxidized to keto acids by removal of the amino group, which is fed to the urea cycle. It is then the keto acid which enters the citric acid cycle and contributes to ATP production.
When there is no oxygen (anaerobic conditions), less ATP is produced. The glycolysis cycle produces lactate, through the enzyme lactate dehydrogenase, which re-oxidizes NADH to NAD+ for re-use in glycolysis. Fats can be broken down to glycerol, which enters glycolysis.
Key biomolecules
While proteins, carbohydrates, and fats are important sources for catabolic reactions, they are also needed for various other functions around the body. Some are also produced through anabolism, in addition to DNA. Minerals are also important for metabolic purposes.
Proteins
Proteins are made of amino acids. During the process of protein synthesis, the amino acids are linked in long chains called polypeptide chains. These are joined together by peptide bonds. The polypeptide chains undergo further modification to form proteins.
Some proteins are used to form the structure of the cells and tissues, while many others are enzymes that catalyze various chemical reactions in the body. Proteins are also important in cell signaling, immune responses, cell adhesion, active transport across membranes, and the cell cycle.
Coenzymes are non- proteins (like minerals or metals) that mediate several chemical reactions in the metabolic pathways of the body. These fall under a few basic types of reactions that involve the transfer of functional groups.
Coenzymes help in the transfer of energy as well. One central coenzyme is adenosine triphosphate (ATP), the energy currency of cells. There is only a small amount of ATP in cells, but it is continuously regenerated. Others include nicotinamide adenine dinucleotide (NADH), a derivative of vitamin B3 that acts as a hydrogen acceptor.
Hundreds of separate types of dehydrogenases remove electrons from their substrates and reduce NAD+ into NADH. This reduced form of the coenzyme is then a substrate for any of the reductases in the cell that need to reduce their substrates. NADH exists in two related forms in the cell, NADH and NADPH. The NAD+/NADH form is more important in catabolic reactions, whereas NADP+/NADPH are used in anabolic reactions.
Carbohydrates
Carbohydrates provide the basic source of energy in the body. Carbohydrates are straight-chain aldehydes or ketones with hydroxyl groups that can exist as straight chains or rings.
Carbohydrates are abundant in nature and play several roles in living organisms. They can be converted to glycogens and used as storage sources of energy as structural components (cellulose in plants, chitin in animals) and as direct source of energy (glucose).
Lipids
Lipids are important biochemicals that have a versatile function in the body. They form the structural part of the biological membranes, such as the cell membrane, or are used as a source of energy. The fats are a large group of compounds that contain fatty acids and glycerol. Production often takes the form of steroids, such as cholesterol, are another major class of lipids that are made in cells.
Lipids and fats illustration. Credit: Naeblys / Shutterstock
Nucleotides
Nucleotides help in the formation of DNA and RNA. DNA and RNA are long chains of nucleotides critical for the storage and use of genetic information. RNA and DNA also code for protein synthesis. Furthermore, nucleotides can act as coenzymes in metabolic group transfer reactions.
Cofactors and minerals in metabolism
The organic compounds (proteins, lipids and carbohydrates) contain the majority of the carbon and nitrogen in humans, whereas most of the oxygen and hydrogen is present in the form of water.
There are several minerals and vitamins that play critical roles in metabolism. Common and abundant among these are sodium and potassium. Other important minerals include calcium, phosphorus, iron, chloride ions, copper, zinc, fluorine, iodine, and magnesium. The metal micronutrients are taken up into organisms by specific transporters.
Cations often act as cofactors that are bound tightly to a specific protein. Enzyme cofactors can be modified during catalysis but cofactors always return to their original state after catalysis has taken place.
Thermodynamics of metabolism
Metabolic processes are chemical reactions, and these often involve generation of heat. Cellular metabolism couples the spontaneous processes of catabolism with the non-spontaneous processes of anabolism. In thermodynamic terms, metabolism maintains the balance.
Chemical reactions are classified as being either exergonic or endergonic. That means that a reaction can either release energy useful for work (an exergonic reaction) or requires energy to proceed (an endergonic reaction). The production of ATP during catabolism is therefore exergonic, whereas anabolism is an endergonic reaction.
Metabolism control
The metabolic pathways are complex and interdependent. With changing environments, the reactions of metabolism must be finely regulated to maintain a constant set of conditions within cells – a condition called homeostasis. Control of metabolic pathways also allows organisms to respond to signals and interact with their environments.
Levels of metabolic regulation
There are multiple levels of metabolic regulation. For intrinsic regulation of metabolic pathways, the reactions self-regulate to respond to changes in the levels of substrates or products. For example, a decrease in the amount of product can increase the metabolic pathway. This is called a feedback mechanism.
Extrinsic control involves a cell in a multicellular organism changing its metabolism in response to signals from other cells. The signals approach the pathways via soluble messengers, such as hormones and growth factors. For example, the hormone insulin from the beta cells of the pancreas is produced in response to rises in blood glucose levels. Binding of the hormone to insulin receptors on cells then activates a cascade of protein kinases that cause the cells to take up glucose and convert it into storage molecules, such as fatty acids and glycogen.
Regulation of carbohydrate metabolism
Glucose homeostasis is a complicated interaction of metabolic pathways, but it’s vital for living organisms. These processes either increase or decrease the blood glucose concentration but they work together in order to maintain an optimal level.
Glucose is derived from carbohydrates taken in the diet. Carbohydrate is digested to the simple sugars: glucose, fructose and galactose. These sugars are absorbed in the intestine and transported to the liver via the portal vein. Thereafter the liver converts fructose and galactose into glucose. Rising levels of glucose in the blood stimulate the release of insulin from the beta cells of the islets of Langerhans in the pancreas.
Insulin is the only hormone that reduces blood glucose levels, and it does this by activating the glucose transport mechanisms and glucose-utilizing metabolic pathways in different tissues of the body. Thus, insulin down-regulates glucose forming pathways.
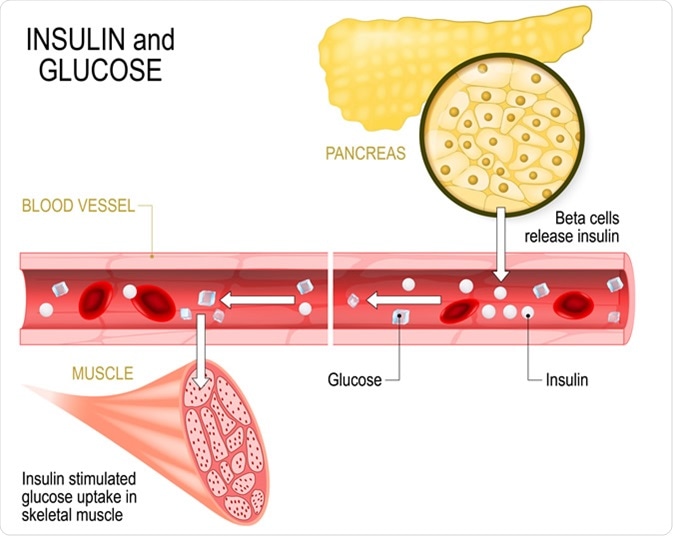
Insulin and glucose. Beta-cells (in the pancreas) release insulin in the blood vessel. Insulin stimulates the absorption of glucose in skeletal muscle. Closeup of pancreas and islets of Langerhans. Image Credit: Designua / Shutterstock
Insulin stimulates glucose uptake (by muscle and adipose tissue), glycolysis, glycogenesis (formation of glycogen from free glucose), and protein synthesis. Conversely, insulin inhibits gluconeogenesis (formation of glucose from fatty acids, etc.), lipolysis (breakdown of fatty acids), proteolysis (breakdown of proteins), and ketogenesis (formation of ketone bodies).
Metabolic disorders and manipulation
Metabolic pathways are complex and often interdependent. Any change in the pathways can give rise to complex disorders. For example, imbalance of the glucose homeostasis and carbohydrate metabolism is linked to diabetes. This makes investigation of metabolic pathways and manipulating them important in clinical diagnosis and management.
Investigations of metabolic pathways and disorders
One of the most helpful tools to investigate imbalanced metabolic pathways is assessment of a pathways’ end products. For example, in diabetes mellitus there is a lack of the hormone insulin that maintains the normal blood sugar, and assessment of fasting (after 8 to 10 hours of no food) and post prandial (2 hours after intake of food) blood sugar helps in diagnosis.
Other methods by which metabolic pathways can be investigated in research (but not clinical care) are through the use of radioactive tracers or metabolomics. Radioactive tracers can help define the paths from precursors to final products by identifying radioactively labelled intermediates and products. Once the tagged chemicals are assessed, the enzymes that catalyze these chemical reactions can be purified and their kinetics and responses to inhibitors can be investigated. Metabolomic studies can provide information about the structure and function of simple metabolic pathways. However, these studies may be inadequate when applied to more complex systems, such as the metabolism of a complete cell. This is because the metabolic networks within the cell contain thousands of different enzymes and complex networks. Genomes reveal that there are nearly 45 000 genes that may code for enzymes and other cofactors within the metabolic pathways.
Manipulation of metabolic pathways
Since the advent of genomic studies, manipulation of gene expression from proteomic and DNA microarray studies have been developed. Many of the inborn metabolic disorders have been treated with gene therapy and manipulation of genes coding for faulty enzymes and proteins in the metabolic pathways.
Using genetics, a model of human metabolism has now been produced, which will guide future drug discovery and biochemical research. These models are now being used in network analysis, to classify human diseases into groups that share common proteins or metabolites.
Metabolic engineering is the targeted and purposeful alteration of metabolic pathways found in an organism. This helps in understanding and utilizing the cellular pathways for chemical transformation, energy transduction, and supramolecular assembly. Metabolic engineering draws principles from chemical engineering, computational sciences, biochemistry, and molecular biology to design and analyze pathways.
Metabolic engineering uses organisms such as yeast, plants or bacteria that are genetically modified to make them more useful in biotechnology and aid the production of drugs, such as antibiotics or the industrial chemicals 1,3-propanediol and shikimic acid. These modifications are aimed at reducing the amount of energy used to produce the product, increasing yields and reducing the production of wastes.
Evolution
Metabolic pathways include several long and complex molecular and chemical reactions that have been conserved over the course of evolution, such that even the simplest organisms share some common metabolic pathways with complex organisms such as humans.
Retention of these ancient pathways may be the result of these reactions being an optimal solution to their particular metabolic problems. For example, glycolysis and the citric acid cycle produce their end products highly efficiently and in a minimal number of steps. This economy and optimal situation has led to the evolution of these reactions over time.
Evolution of the citric acid cycle
The evolutionary origin of the citric acid cycle has long been a model case in the understanding of the origin and evolution of metabolic pathways. Although the chemical steps of the cycle are preserved intact throughout nature, diverse organisms make diverse use of its chemistry. In some cases, organisms use only selected portions of the cycle.
More than one hypothesis has been proposed to explain the evolution of metabolic pathways. These include the sequential addition of novel enzymes to a much shorter earlier pathways, as well as the recruitment of pre-existing enzymes and their assembly into a novel reaction pathway.
Genomic studies have shown that enzymes in a pathway are likely to have a shared ancestry, suggesting that many pathways have evolved in a step-by-step fashion. Along the development of the pathways, novel functions were created from pre-existing steps in the pathway.
An alternative hypothesis comes from studies that trace the evolution of proteins' structures in metabolic networks. This shows that enzymes are pervasively recruited. This recruitment processes result in an evolutionary enzymatic mosaic.
There is also a possibility that some parts of metabolism might exist as "modules" that can be reused in different pathways and perform similar functions on different molecules. Additionally, some of the functions and parts of the pathways that are not essential for survival are lost over tim.
History
Metabolism and metabolic pathways have been studied over several centuries and has moved from examining whole animals in early studies, to examining individual metabolic reactions in modern biochemistry and molecular biology.
Early metabolic studies
Metabolic studies have been conducted as early as the thirteenth century by Ibn al-Nafis (1213-1288), who stated that "the body and its parts are in a continuous state of dissolution and nourishment, so they are inevitably undergoing permanent change."
The original recorded and more sophisticated studies of metabolism began in the closing decades of the sixteenth century. It was during this time that direct observation was augmented by instrumentation that allowed for quantification and, therefore, verification in sciences, especially of biological systems. In medicine, progress depended on the application of the exact sciences of chemistry, mathematics and physics to the study of function.
Santorio Sanctorius (1561- 1636) contributed to metabolic studies by exploring perspiration. His efforts over years of experimentation gave rise to the metabolic balance studies. The first controlled experiments in human metabolism were published by Santorio Santorio in 1614 in his book ''Ars de statica medecina''. In his experiments, he weighed himself before and after eating, sleep, working, sex, fasting, drinking, and excreting. He found that most of the food he took in was lost through what he called "insensible perspiration".
Initial studies of metabolism were conducted on living animals or human volunteers. The mechanisms of these metabolic processes had not yet been identified and a vital force was thought to animate living tissue.
19th century metabolic studies
It was in the 19th century when Louis Pasteur was experimenting with yeast’s fermentation of sugar to alcohol that he noted that fermentation was catalyzed by substances within the yeast cells he called "ferments".
This discovery, along with the publication by Friedrich Wöhler in 1828 of the chemical synthesis of urea, laid the basis for studying organic compounds and chemical reactions found in cells that make up metabolic pathways.
20th century metabolic studies
Eduard Buchner in the beginning of the 20th century advanced the knowledge further by discovering enzymes. He found that the study of the chemical reactions of metabolism was a different branch from the biological study of cells and began to understand the basics of biochemistry. The early 20th century saw rapid development in biochemical studies.
The most notable findings were the discovery of the citric acid cycle by Hans Krebs, who made huge contributions to the study of metabolism. He discovered the urea cycle and later, working with Hans Kornberg, the citric acid cycle and the glyoxylate cycle.
Current metabolic studies
Metabolism is now studied with the help of molecular biotechnology techniques and genomics. Instruments such as chromatography, X-ray diffraction, NMR spectroscopy, radioisotope labelling, electron microscopy and molecular dynamics simulations are commonly used. These techniques have allowed the discovery and detailed analysis of the metabolic pathways and the genetic basis of metabolic disorders.
Studies over the last two centuries have also made advances in the understanding of drug metabolism and metabolism of xenobiotics.
Sources
- Berg, J.M. et al. (2002). Chapter 22 of Biochemistry. 5th Edition. https://www.ncbi.nlm.nih.gov/
- McKee, T., and McKee, J.R. (2015). Chapter 8 of The Molecular Basis of Life. 6th Edition. https://global.oup.com/?cc=in
- Da Poian A.T., et al. (2010). Nutrient Utilization in Humans: Metabolism Pathways. Nature Education. 3(9):11. www.nature.com/.../nutrient-utilization-in-humans-metabolism-pathways-14234029
- Chemistry LibreTexts. The Catabolism of Proteins. (2014). chem.libretexts.org/.../26.09%3A_The_Catabolism_of_Proteins
- Alves, R. et al. (2002). Evolution of Enzymes in Metabolism: A Network Perspective. Journal of Molecular Biology. 324(2):387. https://doi.org/10.1016/S0022-2836(02)00546-6
- Forst, C.V., and Schulten, K. (1999). Evolution of Metabolisms: A New Method for the Comparison of Metabolic Pathways Using Genomics Information. Journal of Computational Biology. 6(3):343-360. https://doi.org/10.1089/106652799318319
- Eknoyan, G. (1999). Santorio Sanctorius – Founding Father of Metabolic Balance Studies. American Journal of Nehprology. 19(2):226-233. https://doi.org/10.1159/000013455
- Manchester, K.L. (1995). Louis Pasteur – Chance and the Prepared Mind. Trends in Biotechnology. 13(12):511-515. https://doi.org/10.1016/S0167-7799(00)89014-9
- Kornberg, H. (2000). Krebs and His Trinity of Cycles. Nature Reviews Molecular Cell Biology. 1(3):225-228. http://www.ncbi.nlm.nih.gov/pubmed/11252898
Further Reading
Last Updated: Mar 11, 2023