Enzymes are essential for life and are one of the most important types of protein in the human body. Studying enzyme kinetics provides information about the diverse range of reactions in the human body, which we can use to understand and predict the metabolism of all living things.
 Image Credit: Maltsev Semion / Shutterstock
Image Credit: Maltsev Semion / Shutterstock
Enzyme-substrate reactions
Before enzyme kinetics can be understood it is essential to understand the basics of what enzymes are and how they work.
Enzymes are natural proteins which acts as catalysts that speed up the rate of specific chemical reactions. They either help create or break down molecules.
The molecules that bind to enzymes are substrates. The mode of action of the enzymes is summarised by the following equation:
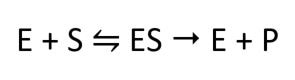
E is the enzyme, S is the substrate, and P is the product formed.
This equation shows that an enzyme and a substrate bind to form an enzyme-substrate complex (ES). This complex then produces the product.
As the enzyme is released at the end and not used up in the reaction, it is a catalyst.
There are many different types of enzymes, and each individual enzyme has an active site with a specific complementary shape for a substrate.
Enzymes bind to these substrates and modify their structure, changing chemical bonds in the substrate to form the product.
An introduction to enzyme kinetics | Chemical Processes | MCAT | Khan Academy
Reaction velocity
The rate of reaction, also called the reaction velocity, is a way of measuring the rate at which the enzyme produces a product.
As the concentration of the substrate increases, the rate of reaction increases, but only up to a certain level called Vmax.
Vmax is the reaction velocity where all enzymes have become saturated, so the velocity cannot increase any more.
We assume that the concentration of enzymes in a reaction is constant, which means that when all the enzymes have a substrate bound to their active sites they are saturated and increasing the concentration of the substrate will have no effect.
The substrate concentration which leads to a reaction velocity of ½ Vmax (half of the maximum reaction velocity) is called Km. Km is inversely proportional to the affinity of the enzyme for the substrate.
In other words, the greater the affinity between enzyme and substrate (and thus lower the Km), a lower concentration of substrate is needed to achieve Vmax.
The Km can be a very useful piece of data to compare the affinity of an enzyme to different substrates. It can also be referred to as the Michaelis constant.
The reaction velocity/ rate of reaction per unit time (V) can be worked out using the Michaelis-Menten equation as follows:
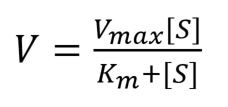
Lineweaver-Burk plot
An easy way to determine Km and Vmax is to plot a Lineweaver-Burk plot. This is a reciprocal graph of the reaction velocity against substrate concentration mentioned earlier.
This means that the y-axis is 1/V and the x-axis is 1/[S].
If you take the reciprocal of the equation to work out reaction velocity and simplify it, then you get the Lineweaver-Burk equation:
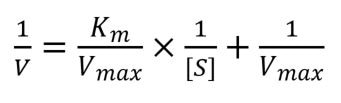
As you can see this equation follows the equation of a line: y = mx + c, which means that it can be plotted easily into a linear graph. Vmax and Km can then be calculated as the y-intercept of the graph equals 1/Vmax and the x-intercept equals -1/Km.
Catalytic efficiency
Another important term in enzyme kinetics is Kcat. This is simply a measure of the efficiency of the enzyme, or how much product it can produce in one second. Kcat is worked out using the equation:
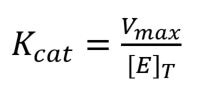
where [E]T is the total concentration of enzyme available for the reaction.
Km and Kcat are therefore both important measures of catalytic efficiency. An enzyme with a high Kcat and a low Km would be an efficient catalyst, whereas an enzyme with a high Km and a low Kcat would not be efficient.
Enzymes (Part 3 of 5) - Lineweaver Burk Plot - Double Reciprocal Plot
Further Reading
Last Updated: Jun 20, 2019