MALDI is the acronym for matrix-assisted laser desorption ionization. It is a type of mass spectrometry imaging that allows for label-free measurements of biological molecules such as proteins and peptides within tissue.
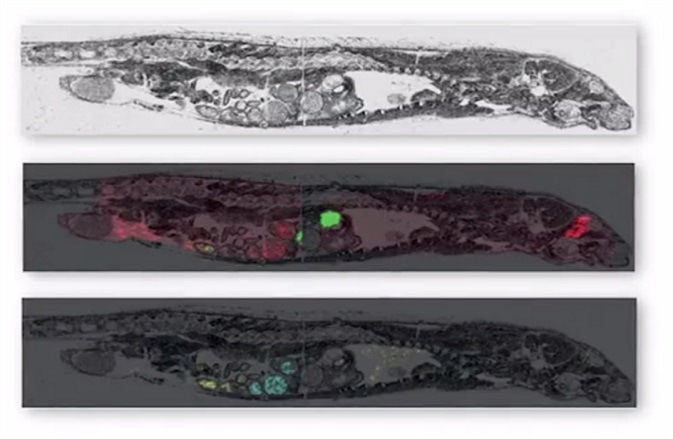 MALDI imaging can simultaneously map hundreds of proteins found in thin tissue sections providing a resolution greater than 25 micrometers. Commercial MALDI imaging technology routinely acquires data with a spatial resolution of between 50 and 200 micrometers. The application of the technology is currently growing with more recent uses being developed in clinical research.
MALDI imaging can simultaneously map hundreds of proteins found in thin tissue sections providing a resolution greater than 25 micrometers. Commercial MALDI imaging technology routinely acquires data with a spatial resolution of between 50 and 200 micrometers. The application of the technology is currently growing with more recent uses being developed in clinical research.
How does MALDI imaging work?
MALDI imaging works through the utilization of a matrix, an acidic aromatic molecule that absorbs energy of the same wavelength produced by the irradiating laser. The matrix transfers the substance being examined to the gas state, thereby producing ionization in a three step process:
- Thin sample sections on a metal slide are first covered with the matrix and the procedure for extracting molecules of interest from the tissue into the matrix begins. The matrix can be applied both manually and automatically.
- The laser irradiates the sample only in the matrix layer, meaning the underlying tissue remains intact.
- The released molecules are transferred to the gas state as the matrix absorbs the laser energy. Ions are formed due to the addition or removal of protons when in the gas state.
The irons are required for further analysis via the mass spectrometer. The metal slide is placed into a MALDI mass spectrometer where the spatial distribution of the biological molecules is mapped. Within the mass spectrometer, the tissue specimen is raster scanned forming a mass spectrum for each spot measured. Image processing software is then required to import the data from the mass spectrometer to allow visualization of the image produced.
Samples are most commonly prepared by freezing, with the sample first wrapped and then submerged in a cryogenic solution. Though images derived from fresh-fixed tissue are the most common, MALDI imaging can also be used on formalin-fixed, paraffin embedded tissues, a preservation method that is frequently applied to archival samples.
Advantages of MALDI imaging
When compared to other technologies for analyzing proteins within tissue, MALDI imaging avoids time consuming extraction, purification and separation steps. Some methods such as liquid-based proteomics also have the ability to obtain similar information, but MALDI imaging has the advantage of producing the information without the loss of spatial distribution data.
The use of the MALDI matrix also means the sample is not destroyed during imaging as the underlining tissue does not come into contact with the irradiating laser and therefore remains intact. Different classes of biological molecule can be chosen for imaging through the selection of the matrix, making the technology suitable for a range of applications.
Applications of MALDI imaging
The technology was first used to map the spatial distribution of proteins. The images produced provided information on the distribution of single proteins as well as posttranslational modification or degradation.
Its application as a method of studying proteomics signatures and biomarkers has increased along with ability to image other molecules in tissue such as metabolites and drugs. Bruker Daltonics is one of the leaders in the field of MALDI imaging. They have developed the MALDI imaging technology that has produced new insights into areas such as the distribution of drugs in tissue and the analysis of cancer samples.
Using MALDI imaging of drugs and metabolites for DMPK
Using MALDI imaging of drugs and metabolites for DMPK from AZoNetwork on Vimeo.
MALDI imaging can differentiate between parent drugs and metabolites within tissue. Knowledge of tissue distribution is important to drug development because it is linked to other drug development areas such as pharmacology and pharmacokinetics. The unintended association of drugs with non-targeted receptors can occur because of accumulation in tissues leading to toxicity.
Additionally, there is no homogenous distribution of drugs and metabolites within the tissue meaning tissue distribution knowledge is particularly important in reducing the risks of toxicity. MALDI imaging has been used to examine tissue distribution for treatments that require the active drug reaching a specific site of infection as well as oncology drugs with specific tumor tissue targets.
Example applications of MALDI imaging in clinical research
MALDI imaging has been utilized in finding a new prognostic marker in breast cancer tissues. HER2 is a receptor that has been defined as a property of breast cancer. When overexpressed, HER2 leads to increased tumor cells.
MALDI imaging was used to determine HER2 status directly from breast cancer tissues. Specific protein/peptide changes in expression were closely linked with HER2 overexpression and the signature was able to distinguish between tissues with a different HER2 status. The study showed the potential for MALDI imaging to be a label-free alternative method for tissue diagnostics.
Another example defined the mechanism behind individual patient responses to cisplatin-based chemotherapy. MALDI imaging was used to detect defects in the mitochondrial respiratory chain, causing the difference in treatment response for advanced adenocarcinoma of the esophagus. The proteomics profiles of patients were analyzed for changes in response to chemotherapy.
The profiles found pre-existing defects that correlated with chemosensitivity. Therefore, MALDI imaging may provide proteomic profiles useful for predicting chemotherapy response in future patients.
Sources:
- Walch, A. et al. 2008. MALDI imaging mass spectrometry for direct tissue analysis: a new frontier for molecular histology, Histochemistry and Cell Biology, 130, pp. 421-434. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2522327/
- National Research Resource for Imaging Mass Spectrometry medschool.vanderbilt.edu/.../high-spatial-resolution-imaging-mass-spectrometry
- Aichler, M. and Walch, A. 2015. MALDI Imaging mass spectrometry: current frontiers and perspectives in pathology research and practice, Laboratory Investigation, 95, pp. 422-432. http://www.nature.com/labinvest/journal/v95/n4/full/labinvest2014156a.html
- Bruker- MALDI Imaging www.bruker.com/applications/life-sciences/maldi-imaging/overview.html
- Castellino, S. et al. 2011. MALDI imaging mass spectrometry: bridging biology and chemistry in drug development, Bioanalysis, 3, pp. 2427-2441. https://www.future-science.com/doi/full/10.4155/bio.11.232
- Rauser, S. et al. 2010. Classification of HER2 Receptor Status in Breast Cancer Tissues by MALDI Imaging Mass Spectrometry, Journal of Proteome Research, 9, pp. 1854-1863. http://pubs.acs.org/doi/abs/10.1021/pr901008d
- Aichler, M. et al. 2013. Clinical response to chemotherapy in oesophageal adenocarcinoma patients is linked to defects in mitochondria, Journal of Pathology, 230, pp. 410-419. https://www.ncbi.nlm.nih.gov/pubmed/23592244
Further Reading
Last Updated: Feb 26, 2019