It is often thought that functions of the human immune defense system are confined to white blood cells (WBCs). However, phagocytes, leukocytes, and macrophages, all of which circulate in our bloodstreams, are not the only cells playing defensive roles.
What is often overlooked is the potential of red blood cells (RBCs) to defend the body against pathogenic invaders. This protective effect of RBCs has been demonstrated against the parasitic species of Plasmodium that causes the blood-borne disease malaria.
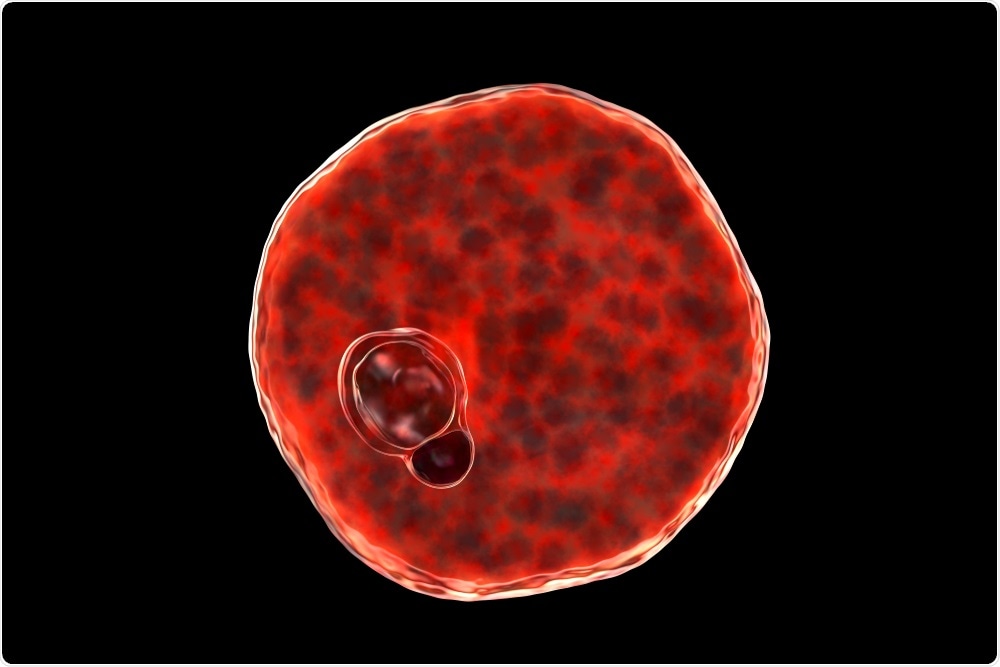
Plasmodium vivax inside red blood cell. Image Credit: Kateryna Kon/Shutterstock.com
An overview of malaria infection
The two parasitic species that are responsible for a majority of malaria cases and deaths include Plasmodium falciparum (P. falciparum) and P. vivax. Briefly, a malaria infection begins with a female Anopheles mosquito that has been previously infected by either parasite bites an individual.
This bite causes the mosquito to release Plasmodium sporozoites, which are the motile and infective form of the parasite, into the affected individual’s skin. Once here, the sporozoites enter and travel through the circulatory system until reaching the liver.
Once in the liver, the Plasmodium sporozoites undergo intrahepatic development to become mature merozoites. These mature merozoites enter a cyclic blood stage where they continuously infect RBCs, thus leading to the various signs and symptoms associated with this infection.
By maintaining much of their existence within the enclosed environment of the RBCs, the parasite is protected against any host immune response that would otherwise lead to their demise. It is only when the parasite is present outside of the RBC, which can occur during traversal or immediately prior to host cell invasion, that it is vulnerable to attack by the host’s immune system.
Type O blood and malaria protection
Cases of sickle cell anemia are concentrated in the African Malaria Belt, where malaria is endemic. This is because the condition offers protection from Malaria, owing to the physical alterations to red blood cell shape and structure.
The types and structures of the molecules coating cell surfaces, which protrude and interact with molecules on other cells, can also affect the ability of parasites to invade cells and therefore establish an infection. The specific features of these molecules define blood groups, the most famous of which would be the ABO± typing system that is heavily relied upon for blood transfusions.
The universal donor blood type is the blood group O. Individuals with type O blood can donate their blood to anyone because of a lack of antigens present in their RBCs. However, type O individuals can only accept blood from other type O donors, as these individuals have both anti-A and anti-B antibodies present within their plasma.
During the late eighties and into the early nineties, researchers found that individuals with type O blood are protected from severe or fatal cases of malaria.
One mechanism that was originally proposed to be responsible for this protection exerted by type O RBCs is known as rosetting. Rosetting refers to the process by which uninfected RBCs adhere to other RBCs that have been infected with P. falciparum parasites. This adherence leads to the formation of rosettes, which prevents the immune system from destroying these cells, as they are surrounded by uninfected RBCs that the immune system will recognize as self.
More recently, the same group of researchers who proposed this resetting mechanism described polypeptide proteins called the repetitive interspersed family of proteins (RIFINs). RIFINs, which are secreted by parasites, allow for the movement of these pathogens toward the surface of infected RBCs.
Once present on the infected cells, the RBCs become sticky and bind together, creating a clot that blocks the blood vessels and contributes to some of the severe symptoms of malaria. Interestingly, RIFINs have been found to bind more strongly to type A RBCs as compared to type O RBCs.
While there is contention over the evolution of the ABO blood groups, some evolutionary biologists hold that O blood types acted as the progenitor of the other subgroups. The predominance of this blood type within malaria-stricken areas may suggest an evolutionary component stemming from the pressure created by the parasite.
In fact, throughout much of Africa, almost half of the population has the blood type O, which is comparable to the United States, in which only about 6.6% of this nation’s population has type O blood.
The Dantu polymorphism
In addition to the protective action of the O blood group, the Dantu Hybrid Rearrangement stands out as another interesting alteration from what would be considered the “norm” for a red blood cell.
As a trait mostly confined to Eastern African countries, it confers up to a 74% reduction in the risk of severe malaria in individuals who are homozygous for this variant. Recent studies have found that the Dantu polymorphism causes extensive changes to arise in several different proteins found on the surface of RBCs.
Specifically, the expression of the glycophorin A (GYPA) intracellular domain was found to be altered in Dantu-individuals, which is significant, as GYPA plays a role in the invasion of P. falciparum merozoites into RBCs. Another observation in this study was that membrane tension is increased in Dantu RBCs, which might cause a greater proportion of the RBCs in these individuals to resist parasitic invasion.
Image Credit: Kateryna Kon/Shutterstock.com
Conclusion
Although the debate continues, it is irrefutable that there is a complex interplay between host and parasite development. This is undoubtedly impacting the structure of red blood cells over the course of progressive generations. It is interesting to consider the many factors which influence variations between human populations. As a species, we continue to evolve alongside other organisms which may be threats or competitors, but potentially spur our own development in a dynamic and complex interplay.
Sources
- Mohandas, N. and An, X. (2012). Malaria and human red blood cells. Medical Microbiology and Immunology, 201(4), pp.593-598.
- WOOD, C., HARRISON, G., DORÉ, C. and WEINER, J. (1972). Selective Feeding of Anopheles gambiae according to ABO Blood Group Status. Nature, 239(5368), pp.165-165.
- Rowe, J., Opi, D. and Williams, T. (2009). Blood groups and malaria: fresh insights into pathogenesis and identification of targets for intervention. Current Opinion in Hematology, 16(6), pp.480-487.
- Farhud, D. and Zarif Yeganeh, M. (2019). A Brief History of Human Blood Groups. Iran Journal of Public Health, 42(1), pp.1-6.
- Leffler, E., et al. (2017). (2017). Resistance to malaria through structural variation of red blood cell invasion receptors. Science, 356(6343), p.eaam6393.
- Carvalho, G. and Carvalho, G. (2011). Duffy blood group system and the malaria adaptation process in humans. Revista Brasileira de Hematologia e Hemoterapia, 33(1), pp.55-64.
- Langhi, D. and Orlando Bordin, J. (2006). Duffy blood group and malaria. Hematology, 11(5-6), pp.389-398.
- Mercereau-Puijalon, O. and Ménard, D. (2010). Plasmodium vivax and the Duffy antigen: A paradigm revisited. Transfusion Clinique et Biologique, 17(3), pp.176-183.
Further Reading
Last Updated: Apr 19, 2021