Pyroptosis is an inflammatory form of programmed cell death that occurs during microbial infections. Different intracellular signaling cascades achieve pyroptosis.
 ellepigrafica | Shutterstock
ellepigrafica | Shutterstock
Why does pyroptosis take place?
Pyroptosis is a form of programmed cell death that occurs during intracellular pathogen infection, as part of the body's antimicrobial response.
Sensing pathogenic infections
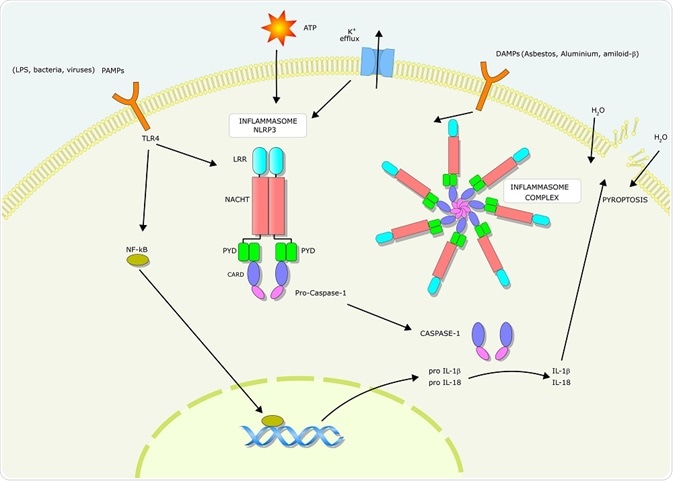
Cell-surface receptors called Toll-like receptors (TLRs) and Nod-like receptors (NLRs) recognize epitopes on the surface of pathogens and microbes. These receptors are mainly expressed by cells of the innate immune system, and play an important role in the body's first defence against pathogens.
TLRs initiate a signaling cascade that results in the releaseof inflammatory cytokines such as tumor necrosis factor (TNF) interleukin (IL)-6, and IL-8. NLRs recognise danger signals within the host cell cytosol and also produces inflammatory cytokines through a different signaling cascade to TLRs. The inflammatory cytokines produced activate the pyroptosis pathway.
The pyroptosis pathway
Pyroptosis is activated through the action of caspase enzymes. In humans, these caspases are activated within an inflammasome, a protein complex composed of inflammasome-initiating sensors and inflammatory caspases. In some cases, an inflammasome adaptor protein (ASC) may also be present.
The formation of the inflammasome is initiated when NLRs recognize danger signals. Certain NLRs (e.g. NLRP3) bind to ASC, which contains a caspase activation and recruitment domain (CARD) which then interacts with caspase-1. Other NLRs (e.g. NLRC4) contain a CARD and do not need to bind to ASC to interact with caspase-1.
Activation of the inflammasome-associated inflammatory caspases cleaves the pro-pyroptotic factor gasdermin D. This produces an N-terminal fragment that oligomerizes to form pores on the host cell membrane. These pores ultimately lead to cell lysis.
Pyroptosis is regulated through caspase-1-dependent and independent mechanisms. Activation of caspase-1, 4, 5, or 11 can form N-terminal fragments, which creates pores. The activation of caspase-1 also causes maturation of IL-1β and IL-18, both of which are involved with inflammasome activation and other immune-related processes.
IL-1β is a pyrogen that causes fevers, leukocyte migration, and expression of various cytokines and chemokines. IL-18 induces the production of IFNγ which is important for the activation of effector T-cells and natural killer (NK) cells.
During pyroptosis, DNA cleavage can occur due to the action of a caspase-1-activated nuclease. Destruction of the actin cytoskeleton is observed in cells undergoing pyroptosis, and caspase-1 inactivates several metabolic proteins during pyroptosis. These changes arrest the homeostasis of the cell and contribute to cell lysis.
Cell repair and survival
Caspase-1 activation does not activate pyroptosis in all cells. For instance, epithelial cells use caspase-1 activation to prevent cell death; lipid production and membrane repair are stimulated by caspase-1. The level of caspase-1 activation regulates the response to microbial stimuli leading to an inflammatory response.
Low levels of caspase-1 stimulate the survival of certain cells, control intracellular bacteria growth, and mediate inflammatory cytokine production. Once the level of caspase-1 activation passes a critical threshold, pyroptosis is initiated.
Summary and conclusion
Pyroptosis is a complex process contributed by different signaling cascades. Research has provided a lot of detail about what triggers pyroptosis, the intracellular signaling pathways involved, and the outcomes of signaling on different cell types.
It is not very clear as to how the inflammatory caspases during pyroptosis are involved in cell repair and survival. Future research on pyroptosis will allow for the manipulation of the pathway for medicine and antimicrobial treatments.
Further Reading
Last Updated: Jan 24, 2019