Skip to:
Oxidative stress occurs when there are high levels of reactive oxygen species (ROS) in the body, that are capable of damaging lipids, proteins, and DNA. Oxidative stress can occur as a result of a number of diseases and conditions, such as in tumors, where oxidized species may interact with tumor suppressor protein p53.
p53 tumor suppressor protein. Image Credit: Juan Gaertner / Shutterstock
Reactive Oxygen Species
Reactive oxygen species (ROS) are a group of compounds that are generated during aerobic metabolism. These compounds include the superoxide anion (O2-), hydrogen peroxide (H2O2), and hydroxyl radicals (OH˙), which all target different molecules. While ROS are commonly linked to damaging molecules and a damaging aspect of pathologies, they can also be signaling molecules used in regulating normal body processes.
Production of ROS comes from both internal and external sources, but mostly occur in atmospheres rich in oxygen. Internal ROS sources include metabolic enzymes and electron leakage from the electron transport chain in the mitochondria. Externally, ROS can be derived from UV light, chemicals, and γ-radiation.
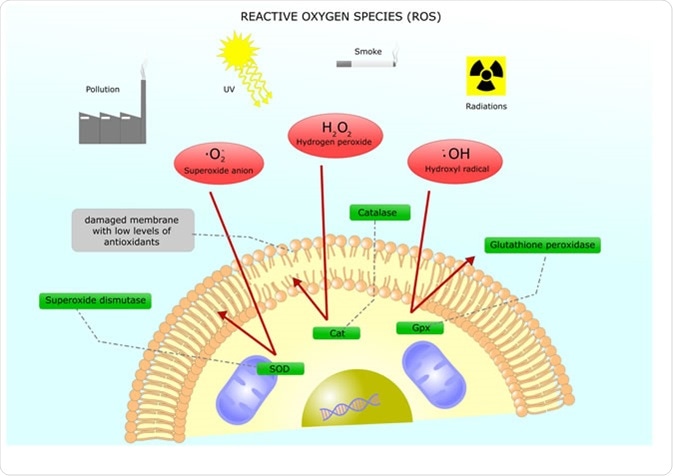
Main reactive oxygen species and their inhibition through antioxidants Image Credit: ellepigrafica / Shutterstock
p53 – The Tumor Suppressor
p53 induces repair or apoptosis in cells with damaged DNA, such as those that have been damaged by ROS. Overall, it is known that p53 has a major role in upholding the integrity of the genome, but finer details of its function are still under discussion. For example, activated p53 interacts with genes involved in the cell cycle and apoptosis, but may also itself detect DNA damage and there is some evidence it directly physically interacts with DNA repair molecules.
The link between p53 and cancer is quite strong, as might be predicted based on its central role in maintaining the genome. More than half of reported cancers have mutations in the p53 gene. Mutations in the gene are also associated with diminished responses to therapeutic agents.
ROS and p53 Pathway
The link between ROS and p53 is complex. ROS levels are heightened in cancer cells compared to healthy cells due to their hyper-metabolism, and these high ROS levels may be responsible for instability in the genome that leads to tumor formation. However, it may be more likely that the increased instability is due to loss of p53, which would otherwise control genomic stability. As opposing forces in such pathologies, it is hypothesized, with some evidence to support it, that one of p53’s primary objectives is to control ROS levels.
One pathway through which ROS and p53 are linked is related to a genetic mutation occurring commonly as a response to ROS. The mutation, an 8-oxoG residue, pulls down a p53 protein along with two proteins of the base excision repair pathway: hOGG1 and APE. The presence of p53 improves APE and hOGG1’s activity significantly, allowing them to efficiently excise the 8-oxoG residue. Such pathways directly link p53 to ROS damage and confirm them as opposing forces.
Another way through which p53 and ROS are believed to be linked is through p53-manipulated metabolism. For tumor growth to proliferate, there is a balance between healthy p53 levels and high ROS levels. One function by which this may unintentionally occur is through antioxidant production regulated by p53. By regulating antioxidant and metabolism genes rather than cell cycle and apoptosis genes, p53 can aim to reduce ROS by encouraging antioxidant activity, which may cause good tumor conditions. Furthermore, such models complicate cancer therapy as it can be in the patient’s best interest to either lower ROS levels to inhibit signaling or to elevate ROS levels to kill cancer cells.
Some forms of cancer are able to directly control p53 function. Promyelocytic leukemia (PML) modifies the organization of recruiting domains called nuclear bodies. The nuclear bodies in turn recruit p53 proteins and control its abundance and/or function. PML cells can sense and build up ROS, and are not able to properly activate p53 targets. This form of cancer has some antioxidant properties, but also encourages changes induced by ROS and alters cellular traits such as proliferation and metabolism.
Sources
- Achanta G. and Huang P. (2004). Role of p53 in sensing oxidative DNA damage in response to reactive oxygen species-generating agents. Cancer Research. https://doi.org/10.1158/0008-5472.CAN-04-0494
- Schieber M. and Chandel N.S. (2015). ROS function in redox signaling and oxidative stress. Current Biology. https://doi.org/10.1016/j.cub.2014.03.034
- Niwa-Kawakita M., et al. (2017). PML is a ROS sensor activating p53 upon oxidative stress. Journal of Experimental Medicine. https://doi.org/10.1084/jem.20160301
Further Reading
Last Updated: Oct 1, 2019