Spectroscopy is the study of the interactions of light and electromagnetic radiation with matter. Over several decades the field has matured into a range of sophisticated analytical techniques with a wide range of applications, from the minute and detailed study of the structure and composition of materials at an atomic scale, to determining the chemical composition of stars are astronomical distances.
 radiorio | Shutterstock
radiorio | Shutterstock
Early Spectroscopy
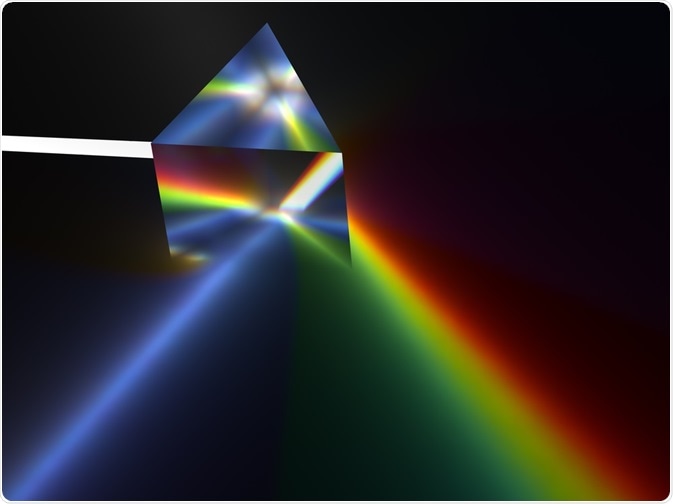
 Ancient Romans were aware that glass prisms are able to separate white light into its constituent colors and generate a rainbow, though they had no conception of the reasons behind this phenomenon at the time.
Ancient Romans were aware that glass prisms are able to separate white light into its constituent colors and generate a rainbow, though they had no conception of the reasons behind this phenomenon at the time.
During the 17th century, several scientists began to study the interaction between light and matter more deeply, including Anglo-Irishman Robert Boyle, and more famously Isaac Newton.
It was Newton who first concluded that when white light is cast through a prism to generate colored light, the white light is divided, rather than the colored light being generated by some property of the prism itself.
Spectroscopy in the 19th Century
The first spectrometer was built by English chemist William Hyde Wollaston in 1802. He used a lens to focus light from the sun onto a screen, which demonstrated that the colors the light was divided into were not evenly distributed, and also presented unexplained dark patches where no light was cast.
It was later discovered that these dark patches are the result of absorption of light by chemicals in the solar and terrestrial atmosphere, and were named Fraunhofer lines thanks to the improvements made to the spectrometer by Bavarian Scientist Joseph von Fraunhofer. These improvements consisted largely of the addition of diffraction gratings, allowing the wavelength of the cast colored light to be determined.
Two English scientists, John Herschel and William Henry Fox Talbot, helped to understand and expand on the cause of the Fraunhofer lines by working with salts. They burned various salts and found that differently colored flames were generated by different salts, later found to be due to the excitation of electrons that emit light at a specific wavelength when returning to their ground state. Similarly, English Scientist Charles Wheatstone found in 1835 that different metals could be distinguished by the color of the sparks they generate when volatilized.
During the mid-18th century multiple scientists, including Scottish-Irish mathematician William Thompson, First Baron Kelvin, were working on this issue, and also found that excited gasses can also emit a very specific wavelength of light. This work birthed the field of atomic emission spectroscopy, currently used to very precisely determine the chemical composition of a sample.
William and Margaret Huggins, English scientists working in the mid to late 18th century, used these spectroscopic techniques to determine the chemical composition of various astronomical bodies, including stars and nebula, and also were the first to distinguish between distant galaxies and nebula using spectroscopy. The red-shift of light generated by astronomical objects moving away from earth was also observed by them using spectroscopy, allowing them to calculate the axial speed of the star Sirius.
 Jurik Peter | Shutterstock
Jurik Peter | Shutterstock
James Clerk Maxwell was a Scottish scientist who summarized and carefully reviewed a great deal of past work on light and spectroscopy, in addition to performing his own experiments, allowing him to write a series of equations that describe electromagnetism. This is considered among the most important work ever completed in the field of physics, along with the work of Isaac Newton and Albert Einstein.
Further Reading
Last Updated: Mar 12, 2019