Thin layer chromatography is used to analyse mixtures: determine the number, identity, and purity of compounds present in a mixture. This technique can also be used to visualize the progress of a reaction.
Thin layer chromatography. Image Credit: Sruilk / Shutterstock
Adsorption Chromatography
Thin layer chromatography is based on the principle of adsorption chromatography. Adsorption refers to a phenomenon where a substance accumulates on the surface of another material, forming a thin layer. This layer is often only one molecule thick. The interaction between adsorbent and adsorbate dictates the separation of the compounds.
Stationary Phase
This phase refers to a layer of adsorbent which is coated on a plate. The adsorbent is selected based on its chemical composition, separation efficiency, particle size, particle size distribution and morphology. Common stationary phases include silica gel, alumina, cellulose powder, modified celluloses, sephadex gels, and kieselguhr.
Thin-Layer Chromatography (TLC)
Mobile Phase
Mobile phase refers to the liquid which carries the sample and transports the stationary phase. The separation of the components on the stationary phase depends on how the different compounds adsorb on the stationary phase and their dissolution in the mobile phase. The mobile phase usually consists of an organic solvent and water. Addition of different compounds may increase or decrease the solubility of specific solutes in the mobile phase. For example, acetic acid in the mobile phase can increase the solubility of basic materials, while ammonia can increase the solubility of acids. Despite these parameters, the actual choice of mobile solvent is based a lot on trial and error.
Properties of Eluting Solvent
The eluting solvent should allow selectivity in the absorption of compounds it is used to separate. It should be able to adsorb well on the adsorbent. Different mixtures of solvents can also be used to increase the eluting power.
Process of Thin Layer Chromatography
This procedure involves the following three steps:
Spotting
The sample which has to be analysed is dissolved in a volatile solvent to create a dilute solution. Then, in spotting, a small amount of this solvent is transferred near the edge of a TLC plate.
Development
In this phase, the edge with the solvent spot is kept in pool of developmental solvent. The solvent travels up in the plate and reaches the solute spot. The polar silica gel adheres to the solute, while the interaction of the solvent with the solute tries to move it up as it proceeds. In the end, the polar properties and subsequent interactions between the solute in the spot, solvent, and the plate determines the movement of the spot. If the solvent is more polar than the plate, then the spot may move up from its place. Also, if the solute has different compounds with different polarities, they will move to different sides on the plate.
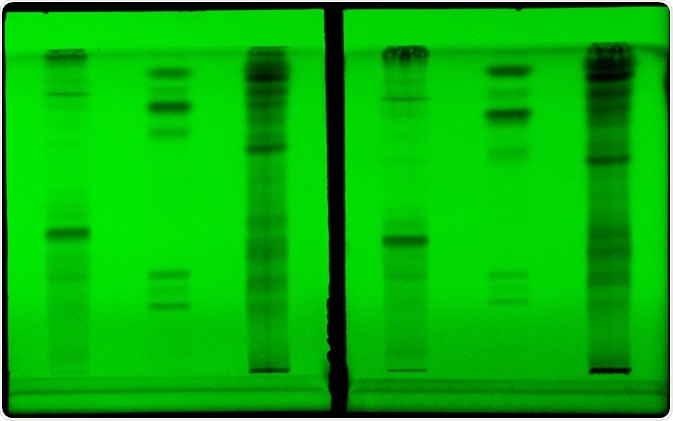
Visualization
Visualization of colored compounds can be simple. After the different compounds have moved to different distances on the plate, they can be directly visualised and analysed. However, visualization of colorless compounds requires further effort. One of the methods is infusing the silica gel on the TLC plate with a fluorescent marker. As the spot moves, it will interfere with the fluorescence of the plate. This will appear as a dark spot or line under the UV light which can mark the position and trajectory. Another method is keeping the plate in iodine vapors. As most organic compounds react with iodine to generate a dark-compound, the spot with organic compounds can be visualised using this method.
Rf Value
This parameter quantifies the movement of solute in thin later chromatography. It gives a measure of the distance travelled by the substance divided by the distance travelled by the solvent. This value ranges between 0 and 1. Another parameter than can be derived is the flow constant. This parameter gives a measure of the migration rate of the solvent. The retardation value can be dependent on the number of factors, including nature of the adsorbent, mobile phase, thickness of the layer, temperature, loading among others.
Further Reading
Last Updated: Sep 17, 2018