Antibody structure
Types of antibodies and their roles
IgG - Immune defense and passive immunity
IgM - Early response and complement activation
IgA - Mucosal protection and neonatal defense
IgE - Allergies and parasite defense
IgD - B and T cell regulation
Camelid antibodies
Antibodies in disease research and treatment
Antibodies in diagnostics
Therapeutic applications of antibodies
Sources
References
Further reading
Antibodies, also known as immunoglobulins (IGs), are glycoproteins produced in response to an immune reaction and exhibit high specificity for the antigens that elicit the response.
Antibody structure
An antibody molecule consists of two heavy chains (approximately 50 kDa each) and two light chains (approximately 25 kDa each). These chains are interconnected by disulfide bonds, resulting in a characteristic Y-shaped structure with a total molecular weight of approximately 150 kDa. Each antibody molecule possesses distinct variable and constant regions.
The variable region confers an antibody's antigen-binding specificity. This region contains two fragment antigen binding (Fab) domains, each capable of binding a specific epitope (the part of an antigen recognized by an antigen). Consequently, a single antibody molecule can simultaneously bind two identical epitopes on the same antigen.
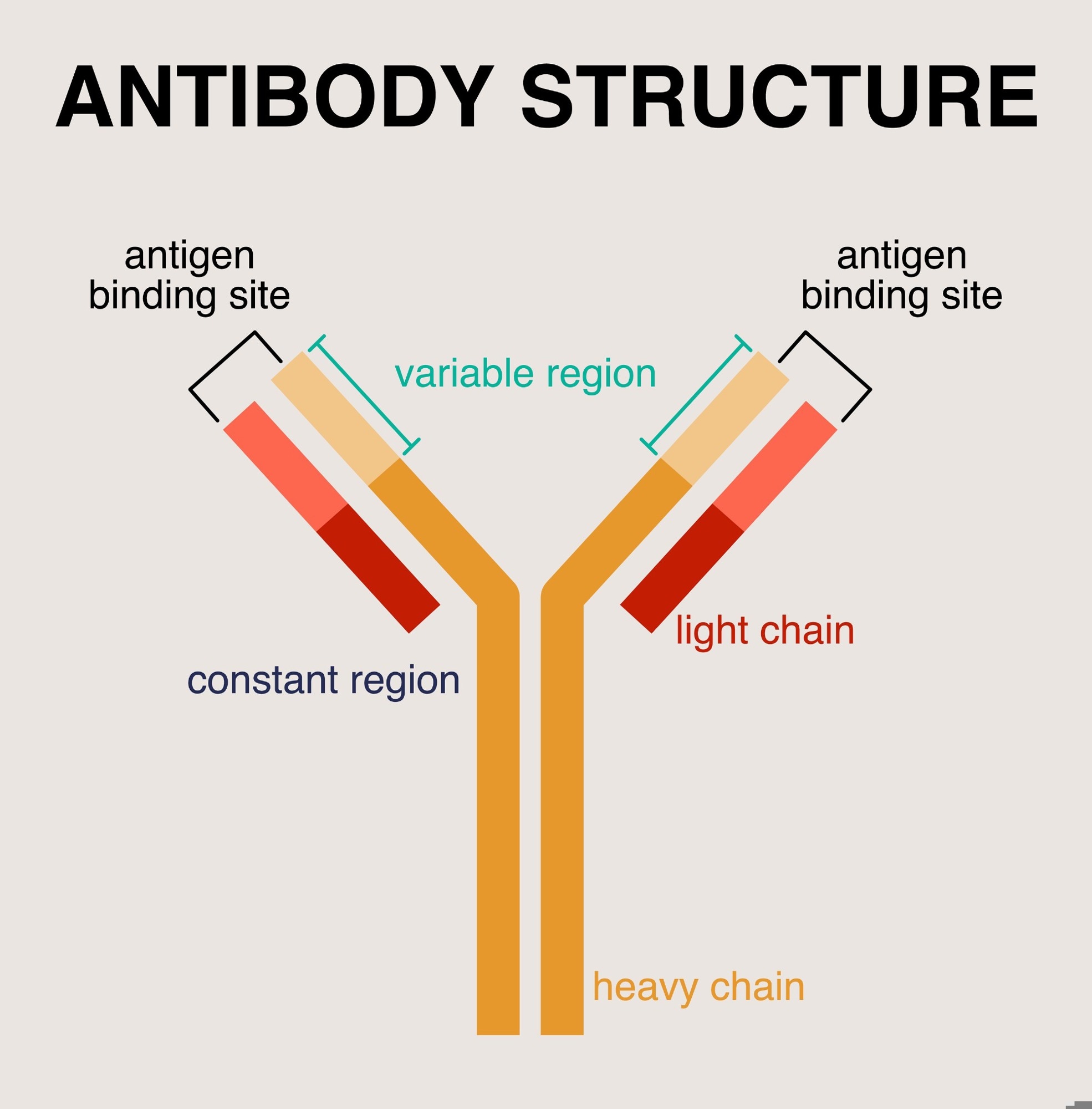 Diagram of an antibody structure showing its key components, including antigen binding sites, variable and constant regions, light chains, and heavy chains. Image Credit: aiyoshi597/Shutterstock.com
Diagram of an antibody structure showing its key components, including antigen binding sites, variable and constant regions, light chains, and heavy chains. Image Credit: aiyoshi597/Shutterstock.com
The constant region of an antibody houses the fragment crystallizable (Fc) region, which mediates interactions with Fc receptors expressed on the surface of immune cells such as leukocytes (white blood cells), macrophages, and natural killer cells. These interactions are crucial for triggering various effector functions of the immune system. Two hinge regions link the Fab and Fc portions of each antibody molecule.
Types of antibodies and their roles
There are five main types (isotypes) of antibodies, each with distinct structures and functions: IgG, IgM, IgA, IgE, and IgD.
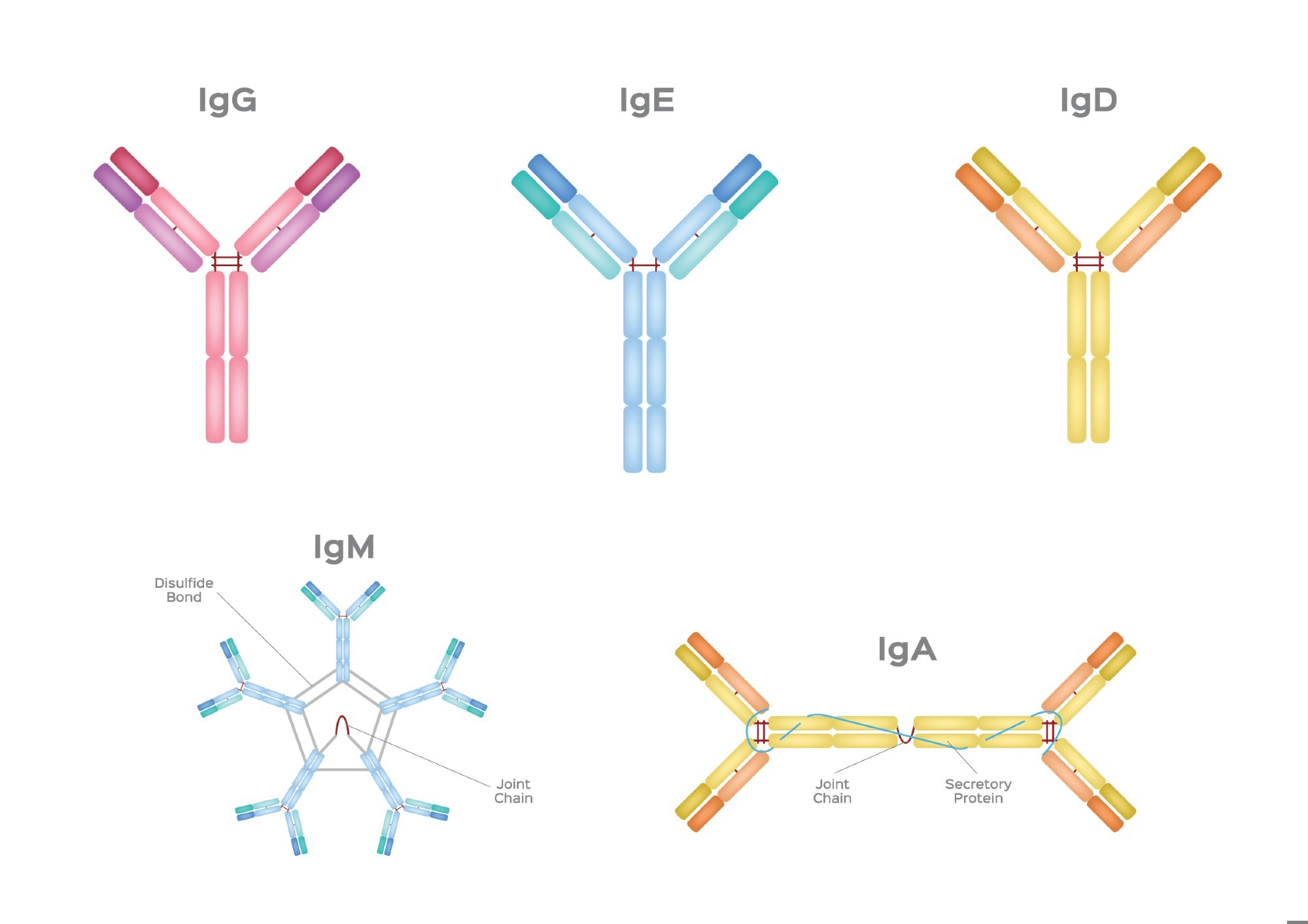 Illustration of the five major antibody (immunoglobulin) types: IgG, IgE, IgD, IgM, and IgA, each with its unique structural configuration and functional properties. Image Credit: gritsalak karalak/Shutterstock.com
Illustration of the five major antibody (immunoglobulin) types: IgG, IgE, IgD, IgM, and IgA, each with its unique structural configuration and functional properties. Image Credit: gritsalak karalak/Shutterstock.com
IgG - Immune defense and passive immunity
IgG is the most abundant immunoglobulin in human serum, comprising 70–75% of the total immunoglobulins present in the blood. Structural variations, primarily in the hinge region (size and flexibility) and the number and position of disulfide bonds, lead to four distinct IgG subclasses: IgG1, IgG2, IgG3, and IgG4, each with unique effector functions.
Protein antigens typically elicit IgG1 and IgG3 responses, whereas IgG2 and IgG4 are generally associated with responses to polysaccharide antigens. IgG's high abundance makes it the principal component of the humoral immune system, which relies on antibodies (immunoglobulins) present in extracellular fluids to neutralize pathogens.
Owing to its small, monomeric structure and high diffusibility, IgG is the most abundant type of immunoglobulin in the extracellular fluid. Its ability to bind Fc receptors on phagocytic or other lytic effector cells enables the initiation of antibody-dependent cell-mediated cytotoxicity (ADCC). ADCC is a cell-mediated immune mechanism whereby effector cells, including phagocytes and natural killer cells, lyse antibody-coated target cells.
IgG promotes phagocytosis by initiating opsonization, a process whereby pathogens (e.g., bacteria) are coated with antibodies, thereby enhancing their recognition and subsequent engulfment by phagocytes.
IgG plays a crucial role in both passive and active immunity. IgG is the only immunoglobulin capable of placental transfer, conferring passive immunity to the developing fetus and neonates for the first few months after birth. Studies have demonstrated the positive impact of heat-treated colostrum on serum IgG concentrations in newborn calves, thereby enhancing passive immunity.1 However, this approach may also have associated side effects.
In contrast, active immunity can be induced through vaccination. For instance, a single dose of a COVID-19 mRNA vaccine may generate high levels of IgG targeting the receptor-binding domain (RBD) of SARS-CoV-2 and elicit surrogate neutralization in individuals with a prior COVID-19 diagnosis.2
IgM - Early response and complement activation
IgM is the largest antibody isotype and the first to be secreted during a primary immune response to an antigen or microbe. It thereby provides immediate immune responses, regulates immune functions, and maintains immunological tolerance.
Representing approximately 5% of serum immunoglobulins, IgM is typically found as a pentamer composed of five identical subunits connected by disulfide bonds and a J chain. This pentameric form is the predominant structure of IgM.
In addition to the pentameric form, IgM also exists as secretory IgM, which is synthesized by plasma cells in mucosal-associated lymphoid tissue (MALT) and other glandular tissues. A monomeric form of IgM is expressed on the surface of B cells, enabling recognition and B-cell activation.
Because of its large size and polymeric structure, IgM is largely confined to the intravascular space and exhibits a lower binding affinity for individual epitopes compared to IgG. Despite this lower affinity, the pentameric form of IgM possesses ten antigen-binding sites, resulting in high avidity (overall binding strength). This high avidity makes IgM a highly efficient excellent activator of the classical complement pathway and a potent inducer of agglutination.
IgM antibodies are frequently used as a diagnostic criterion for acute or recent disease, but unconventional IgM-specific responses have been described in SARS-COV-2 infection.3
IgA - Mucosal protection and neonatal defense
IgA represents 10–15% of the total immunoglobulins in serum and is found in various secretions, including nasal mucus, saliva, breast milk, and intestinal fluid. Two subclasses of IgA exist—IgA1 and IgA2 —which are distinguished primarily by structural differences in their hinge regions.
IgA plays a crucial role in mucosal immunity, providing the first line of defense against pathogens encountered at mucosal surfaces, such as those of the respiratory and gastrointestinal tracts. The importance of IgA in maintaining mucosal immunity is highlighted by the increased susceptibility to infections observed in individuals with IgA deficiency. Studies have shown that IgA is critical for clearing gastrointestinal viral pathogens such as rotavirus and preventing reinfection.4 Furthermore, selective IgA deficiency appears to be a significant risk factor for recurrent chest infections, particularly in asthmatic patients.5
IgE - Allergies and parasite defense
IgE is the least common immunoglobulin in serum, present at concentrations roughly 10,000 times lower than those of IgG in healthy individuals. Clinically, however, IgE levels rise dramatically in type I hypersensitivity reactions, including allergic diseases like bronchopulmonary aspergillosis and parasitic infections such as schistosomiasis.
In response to pathogens, IgE antibodies bound to mast cells through high-affinity receptors undergo cross-linking upon antigen binding. This cross-linking triggers mast cell degranulation, releasing inflammatory mediators that recruit eosinophils to the site of infection. The eosinophil-mediated killing of the pathogen can then occur through mechanisms resembling antibody-dependent cell-mediated cytotoxicity (ADCC).
IgD - B and T cell regulation
IgD is primarily known for its role as a B-cell antigen receptor (BCR) on the surface of B cells. It is thought to participate in various B-cell processes, including maturation, maintenance of peripheral tolerance, activation, and potentially even silencing.
The precise mechanisms and full extent of IgD's functions are still under investigation. Still, current evidence suggests a role in regulating B-cell selection and maintaining B-cell homeostasis, thus influencing humoral immune responses.
 3D illustration of antibodies binding to viral particles, highlighting the immune response in neutralizing pathogens and preventing infection. Image Credit: Anusorn Nakdee/Shutterstock.com
3D illustration of antibodies binding to viral particles, highlighting the immune response in neutralizing pathogens and preventing infection. Image Credit: Anusorn Nakdee/Shutterstock.com
Recently, it has also been suggested that IgD signaling can be a novel therapeutic target for treating T cell-related diseases, such as autoimmune and hematological diseases.6
Camelid antibodies
Camelid heavy-chain antibodies, often referred to as nanobodies, are unique in that they are composed solely of heavy chains and lack light chains, the typical components of conventional antibodies. This distinct structure was first observed in 1989 during the analysis of both total and fractionated IgG from camel serum.
These antibodies possess a unique antigen-binding site consisting of a single variable domain (VHH or nanobody). This single-domain architecture confers several advantages, making them attractive candidates for therapeutic applications. Some advantages include their smaller size, which facilitates tissue penetration; high solubility and thermostability, which enhance their stability and ease of production; high affinity and specificity for target antigens; and low immunogenicity, reducing the risk of adverse immune reactions.
Camelid antibodies have been shown to exhibit enhanced sensitivity and specificity in diagnostic systems for detecting cancer cells, degenerative disease biomarkers, viral antigens, bacterial toxins, and insecticides.7
Antibodies in disease research and treatment
Antibodies have emerged as a crucial and rapidly advancing class of therapeutics for a wide range of diseases, including cancer, autoimmune disorders, infectious diseases, and hematological malignancies. Indeed, therapeutic antibodies represent the fastest-growing class of biological drugs approved for clinical use, demonstrating their significant impact across diverse disease areas, from oncology to autoimmunity, with considerable potential for future development.8 Ongoing innovations in antibody engineering, antibody-drug conjugates, and computational design are further enhancing their efficacy and expanding their therapeutic applications.
Antibodies in diagnostics
Antibody-based assays, such as lateral flow assays and ELISAs, offer valuable tools for diagnostic purposes. Studies have shown that both techniques provide consistent results for measuring IgG and IgM antibodies against SARS-CoV-2 spike and nucleocapsid proteins, suggesting their utility for COVID-19 detection, particularly in settings with limited access to molecular tests.9
Furthermore, IgG/IgM-based ELISA tests demonstrate the highest overall diagnostic accuracy for COVID-19, with combined IgG/IgM detection showing greater sensitivity than measuring either antibody isotype alone.10 However, the choice of antibody type can impact assay performance in other contexts.11
Therapeutic applications of antibodies
Antibody-based therapeutics are now capable of targeting previously undruggable targets, leading to a deeper understanding of biological processes and the development of potential treatments for various diseases.12 One particularly promising area is the targeting of intracellular tumor antigens with therapeutic antibodies, which holds significant potential for clinical translation in cancer therapy.13 The convergence of advances in antibody engineering continues to drive the development of innovative and more effective antibody-based therapies.
References
- Rabaza, A., Fraga, M., Mendoza, A., & Giannitti, F. (2023). A meta-analysis of the effects of colostrum heat-treatment on its viscosity and IgG concentration, and the transfer of passive immunity in newborn dairy calves. Journal of dairy science. https://doi.org/10.3168/jds.2022-22555.
- Demonbreun, A., Sancilio, A., Velez, M., Ryan, D., Saber, R., Vaught, L., Reiser, N., Hsieh, R., D’Aquila, R., Mustanski, B., McNally, E., & McDade, T. (2021). Comparison of IgG and neutralizing antibody responses after one or two doses of COVID-19 mRNA vaccine in previously infected and uninfected individuals. EClinicalMedicine, 38. https://doi.org/10.1016/j.eclinm.2021.101018.
- Fonseca, M., Silva, M., Pinto, A., De Melo, A., Oliveira, F., De C Araújo, F., & De Andrade, L. (2022). Persistently positive SARS‐CoV‐2‐specific IgM during 1‐year follow‐up. Journal of Medical Virology, 94, 4037 - 4039. https://doi.org/10.1002/jmv.27822.
- Blutt, S., Miller, A., Salmon, S., Metzger, D., & Conner, M. (2012). IgA is Important for Clearance and Critical for Protection from Rotavirus Infection. Mucosal immunology, 5, 712 - 719. https://doi.org/10.1038/mi.2012.51.
- Ali, F., Mahmoud, N., El-Sayed, A., Abdelmaksoud, M., Shata, A., & Fouad, S. (2021). Selective IgA Deficiency a Probable Risk of Recurrent Chest Infections in Asthmatics. Journal of Asthma and Allergy, 14, 1323 - 1333. https://doi.org/10.2147/JAA.S329531.
- Wei, W., & Wu, Y. (2019). Innovative drug research targeting IgD and IgDR signaling for the regulation of T cell activity. The FASEB Journal, 33. https://doi.org/10.1096/fasebj.2019.33.1_supplement.670.2.
- Brilhante-Da-Silva, N., De Oliveira Sousa, R., Arruda, A., Santos, E., Marinho, A., Stabeli, R., Fernandes, C., & Pereira, S. (2021). Camelid Single-Domain Antibodies for the Development of Potent Diagnosis Platforms. Molecular Diagnosis & Therapy, 25, 439 - 456. https://doi.org/10.1007/s40291-021-00533-7.
- Sharma, P., Joshi, R., Pritchard, R., Xu, K., & Eicher, M. (2023). Therapeutic Antibodies in Medicine. Molecules, 28. https://doi.org/10.3390/molecules28186438.
- Arıkan, A., Doluca, O., Akhan, S., Şanlıdağ, T., & Sayan, M. (2022). Evaluation of lateral flow and ELISA techniques for detecting IgG and IgM antibodies in COVID-19 cases in Türkiye. Eastern Mediterranean health journal, 29 2, 91-99. https://doi.org/10.26719/emhj.23.011.
- Vengesai, A., Midzi, H., Kasambala, M., Mutandadzi, H., Mduluza-Jokonya, T., Rusakaniko, S., Mutapi, F., Naicker, T., & Mduluza, T. (2020). A systematic and meta-analysis review on the diagnostic accuracy of antibodies in the serological diagnosis of COVID-19. Systematic Reviews, 10. https://doi.org/10.1186/s13643-021-01689-3.
- Qriouet, Z., Cherrah, Y., Sefrioui, H., & Qmichou, Z. (2021). Monoclonal Antibodies Application in Lateral Flow Immunochromatographic Assays for Drugs of Abuse Detection. Molecules, 26. https://doi.org/10.3390/molecules26041058.
- Qian, L., Lin, X., Gao, X., Khan, R., Liao, J., Du, S., Ge, J., Zeng, S., & Yao, S. (2023). The Dawn of a New Era: Targeting the "Undruggables" with Antibody-Based Therapeutics. Chemical reviews. https://doi.org/10.1021/acs.chemrev.2c00915.
- Trenevska, I., Li, D., & Banham, A. (2017). Therapeutic Antibodies against Intracellular Tumor Antigens. Frontiers in Immunology, 8. https://doi.org/10.3389/fimmu.2017.01001.
Further Reading
Last Updated: Feb 3, 2025