Physicians now have access to an even faster and more accurate handheld ultrasound device, with Signostics today unveiling its new “Signos RT” model.
The Signos RT is Signostics’ second generation portable handheld ultrasound device and builds on the cutting-edge technology of the original Signos device by giving users the critical ability to view ultrasound images in real time.
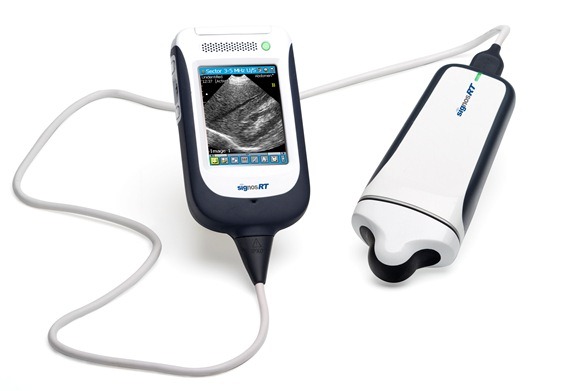
Signostics anticipates the Signos RT will attract widespread interest from medical practitioners, healthcare professionals and veterinarians globally. The product is already available for sale in Australia and Europe, with USA availability for the human market pending 510(k) clearance.
Signostics Chairman Raymond Spencer says development of the Signos RT was made possible by a $1.9 million Early State Commercialisation grant from Commercialisation Australia (awarded in August 2011), which has helped position the company to capture a larger slice of the growing personal ultrasound market currently estimated to be worth around $3 billion globally.
“From humble beginnings, Signostics has already enjoyed tremendous success and is now on the verge of becoming a worldwide leader in fast and affordable point-of-care medical devices,” said Mr Spencer.
“The next-generation Signos RT has the potential to change the future of medicine and allow physicians to see more and do more anywhere at any time.”
Significantly, Signostics has also today announced a key distribution partnership with Konica Minolta Medical & Graphic Inc.
“At Signostics, we have more than just imagination and today we can announce we have signed an agreement with Konica Minolta Medical & Graphic Inc to sell our devices exclusively through Japan, USA, China and India,” said Signostics’ Chief Executive Officer Warren Ortmann.
“We have also received enormous interest from potential distribution partners throughout Europe, South East Asia and our own Australian market who have seen and experienced our prototype device.
“Under the agreement, Signostics will manufacture the Signos RT with the help of our partner suppliers and deliver it exclusively to Konica Minolta Medical & Graphic Inc under their brand, Sonimage P3, to promote and sell via their existing supply channels in both the human and veterinary markets.
“An OEM partnership of this kind will greatly assist our growth and be a real game-changer for Signostics in terms of distribution and international sales.”
The President and CEO of Konica Minolta Medical & Graphic Inc Atsushi Kodama is anticipating an exciting future ahead.
“We are very excited about selling this truly portable and flexible point-of-care imaging tool and incorporating it into our product portfolio,” said Mr Kodama. “Signostics has developed a revolutionary product which we believe will have a significant impact across our global markets.”
South Australian Minister for Manufacturing, Innovation and Trade Tom Kenyon congratulated Signostics on its achievement and praised the company for its commitment to innovation.
“This is a big win for South Australia with the main component of this new ultrasound device to be manufactured right here in Adelaide,” said Mr Kenyon. “Developing niche, high-value products such as this is one is a key objective of South Australia’s Manufacturing Works strategy. It’s a great example of what can be achieved when industry, researchers and government work together.”
Since its launch in 2009, the Signos has been widely distributed among rural and respiratory physicians, physiotherapists, continence nurses and palliative care specialists, as well as companion animal veterinarians. The product is helping medical practitioners to visually assess a patient’s internal anatomy more efficiently than ever before, with the latest innovative technology enabling rapid production of high resolution images to assist clinicians diagnose patients and improve health at a much more affordable price.
ABOUT SIGNOSTICS
Signostics Limited was established in Adelaide, South Australia, in 2005 to be the world leader in fast and affordable point-of-care ultrasound medical devices. After opening its sales and marketing office in Palo Alto, California, the company launched its first product into the veterinarian market
in 2009 before gaining regulatory approvals to enter the human medical device market in Australia, the United States and Europe.
Signos RT features unique patented technology and an award-winning design to provide a completely portable, lightweight, handheld ultrasound device for point-of-care use by medical personnel in a number of clinical settings.
Continuing research, development and commercialisation of the Signos RT device has been aided by the Australian Government through the Commercialisation Australia grant program, as well as the ongoing support of private investors and venture capital funding.
For additional information about Signostics Limited please visit www.signostics.com.au