Dr. Dave Fancy discusses the common challenges that scientists face when developing nanobodies and how anti-alpaca secondary antibodies can be used to produce high-affinity nanobodies and VHH libraries.
In which fields are nanobodies being adopted?
Nanobodies were discovered in the 1990s, but have only been picked up heavily by the research community in the last 10 years. The dramatic rise of interest in nanobodies is observable in 2010, when there was an inflection point for “nanobodies” among search engine hits. Before 2010, there was an average of 200 to 300 new citations per year for nanobodies. As of 2019, this has increased to over 2,000 new citations per year.
 Carl Dupont | Shutterstock
Carl Dupont | Shutterstock
While there are several reasons why nanobodies are rising in popularity, a large contributing factor is that their manufacture has recently come off of patent. Due to this, many researchers, pharmaceutical and diagnostic companies have started to embrace them and are incorporating them into their research pipeline or reagent toolbox.
What are the main features of nanobodies that make them so useful?
There are several key advantages that have led to the adoption of nanobodies:
Firstly, camelids, such as alpaca or llama, produce two subclasses of antibodies, IgG2 and 3. These interesting subclasses are comprised of only two heavy chains, each with a monomeric variable domain (VHH) from which a nanobody is generated.
Secondly, nanobodies have a long 3rd CDR. This makes them well suited for entering epitope “pockets.” Unlike conventional antibodies that comprise of both light and heavy chains, and tend to be flatter or accept convex surfaces, nanobodies can reach into concave surfaces binding to those areas that typical antibodies are poor at. The upshot of this is that epitopes on pathogens or drug targets normally inaccessible to full length antibodies are now open for targeting by a VHH domain antibody.
The final advanatge of nanobodies is their small size. This very appealing pharmacokinetic property allows them to quickly pass through the kidneys and other barriers otherwise inaccessible. Combined with their specificity, this enables payloads, such as drugs or fluorescent molecules, to be attached for targeted delivery in vivo, making them very attractive to pharmaceutical companies.
How are nanobodies produced?
Traditionally, if you want an antibody of consistent quality and in large supply, you have to remove the B-cells from an immunized rodent and make a monoclonal antibody from the hybridoma. For nanobodies however, there are a couple of alternate approaches that are attractive.
The first approach is to simply isolate B-cells from an unimmunized camelid, PCR out the VHH gene segment and create a naïve library expressing the billions of VHH domains on phage. This library contains just the background VHH and has not been biased to anything, however some elaborate high throughput screening and genetic engineering strategies need to be employed if a tight binding nanobody is desired.
The second approach is to isolate B-cells from an immunized llama or alpaca. The challenge with this is knowing the optimal time to harvest the B-cells. The animal goes through the natural process of affinity maturation, which results in antibodies that have been selected to bind to epitopes with higher affinity and specificity.
It’s imperative to monitor the immune response of IgG2 and 3 subclasses (where VHH is found) before you harvest the B-cells. As IgG1 is typically the predominant subclass, the measured total immune response can mislead researchers into thinking an optimal titer has been reached and they might miss the sweet spot for B-cell collection of IgG2 and 3 subclass expressing cells.
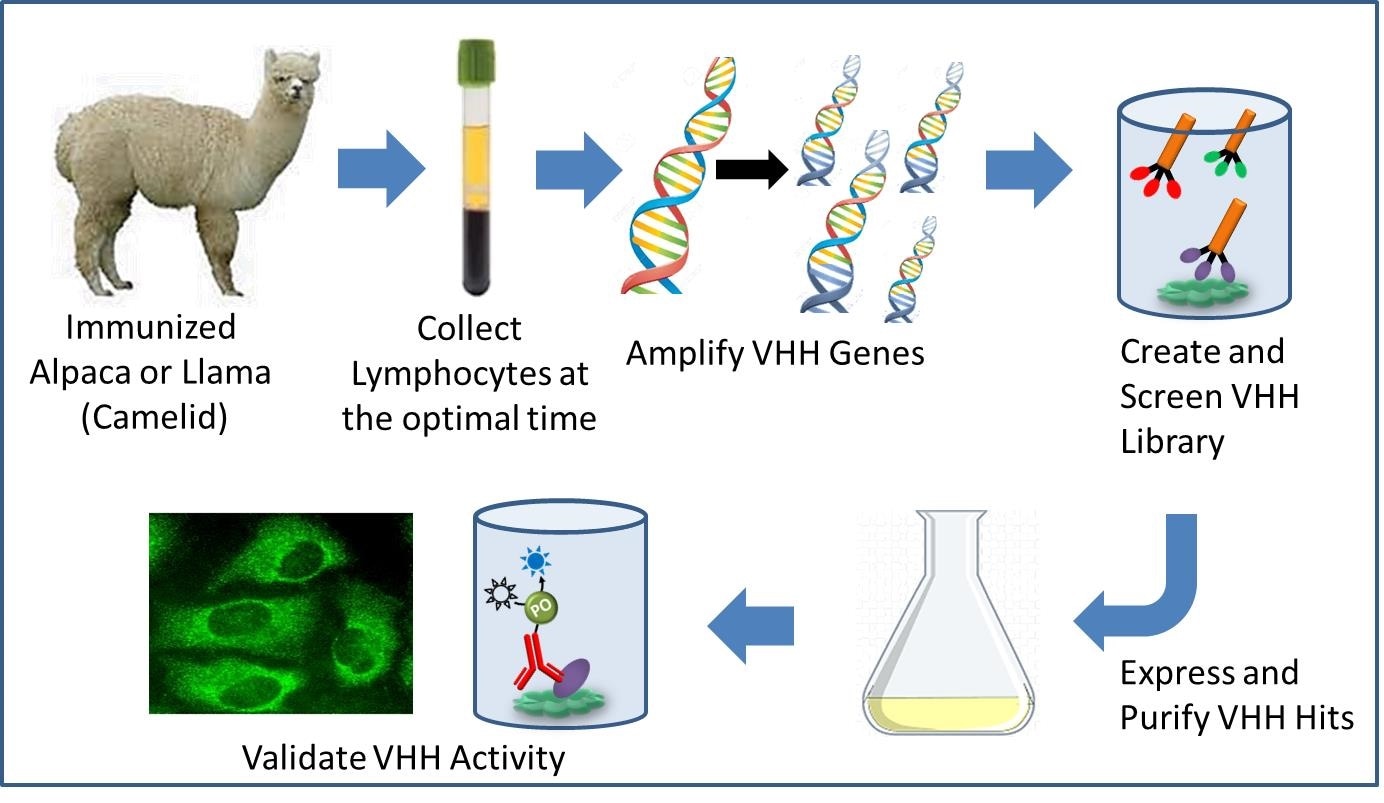 Pathway to recombinant VHH antibody development. (Image provided by Jackson ImmunoResearch).
Pathway to recombinant VHH antibody development. (Image provided by Jackson ImmunoResearch).
How can the new Secondary Antibodies developed by JIR help scientists to identify, develop and produce nanobodies?
To help scientists working with nanobodies, Jackson ImmunoResearch has created a portfolio of products for VHH discovery:
To understand the immune response, we developed secondary antibodies that specifically recognize both IgG2 and IgG3 subclasses, with minimal cross-reactivity to IgG1. This reagent enables customers to start with an immunized animal, sample blood, observe the IgG2 and IgG3 titers, and when desired harvest serum or B-cells.
To resolve issues that typically occur when using monoclonal anti-tag antibodies, where the result can be weak signal or high background, we developed a polyclonal anti-VHH secondary antibody that recognizes multiple epitopes. Many anti-VHH conjugated secondaries can bind a nanobody simultaneously and generate a higher signal.
For complicated multi-labeling experiments using several antibodies from different species, we developed an anti-VHH secondary antibody that has been highly cross-adsorbed that reacts minimally to Bovine, Human, Mouse, Rabbit, and Rat IgGs and serum proteins.
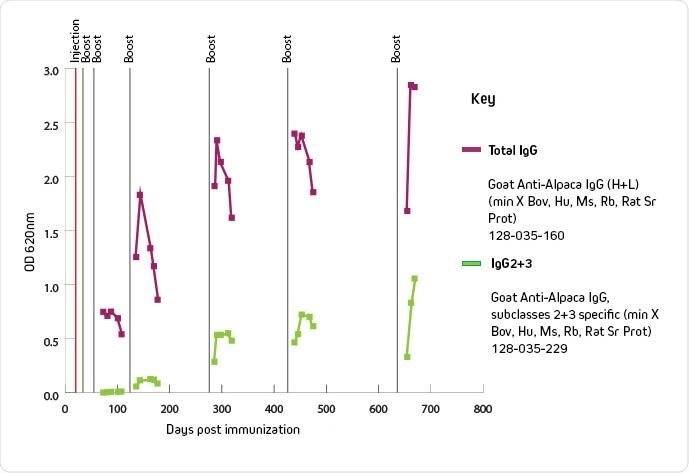 Graph showing IgG 2+3 titer over time. (Image provided by Jackson ImmunoResearch).
Graph showing IgG 2+3 titer over time. (Image provided by Jackson ImmunoResearch).
How can JIR Secondary antibodies for VHH discovery be used to screen and validate nanobodies?
We understand our customers utilize many different screening assays, like flow cytometry and ELISA. Therefore, we packaged our secondary antibodies specific to VHH discovery in large enough quantities at a reasonable price to make them amenable for high throughput screening. They also come in a variety of conjugates that include, but are not limited to: Horseradish peroxidase, Alexa Fluors®, Cy™ dyes, R-Phycoerythrin (R-PE), and Biotin.
What advantages do JIR Secondary Antibodies for VHH discovery offer over existing methods?
Typically, when researchers start expressing VHH domains, they immediately put an epitope tag on them to aid in purification and monitoring. While these tags can be helpful, they can interfere with nanobody binding or limit nanobody detection. Small epitope tags are generally bound by one conjugated antibody so the signal tends to be poor. Depending on the quality of the anti-tag antibody, there can also be a lot of background, which can confound some experiments.
The advantages of Jackson ImmunoResearch’s secondary antibodies for VHH discovery are simple. These reagents are polyclonal and highly specific, allowing for multiple interactions with the VHH domain. Since each secondary antibody contains multiple fluorophores or enzymes, the signal: noise ratio is much better.
What’s next for the company?
Jackson ImmunoResearch continues to explore projects within the nanobody space. We have validated the anti-VHH antibodies in a variety of assays and recently published a whitepaper documenting their performance to help people understand the fundamental benefits they offer. We are also constantly working to improve our antibody specificity and to innovate new products.
As technology continues to advance, instruments become more sensitive, and assays become more complicated, we are determined to meet our customers’ needs as they raise the bar. Researchers need great tools to make great discoveries and Jackson ImmunoResearch sees itself as a partner to all scientists in this endeavor.
About Dr. Dave Fancy
 Dave Fancy is the COO of Jackson ImmunoResearch (JIR), a position he has held for the past 5 years. Prior to his role at JIR, Dave worked as a senior scientist at SDIX, where he focused on the development of polyclonal and monoclonal antibodies for immunoassays.
Dave Fancy is the COO of Jackson ImmunoResearch (JIR), a position he has held for the past 5 years. Prior to his role at JIR, Dave worked as a senior scientist at SDIX, where he focused on the development of polyclonal and monoclonal antibodies for immunoassays.
Dave received a Ph.D. in Organic Chemistry from the University of Texas at Austin in 1997. He then became a postdoctoral research fellow in structural biology at The University of Texas Southwestern Medical Center, and after two years became an Assistant Professor.
In his current role, Dave leads the development and commercialization of antibody-based products and services for researchers working in the life sciences.