Life science companies are increasingly investing in holistic talent management systems (TMS), where learning management presents an important feature. However, the question that arises at this juncture is what exactly does FDA anticipate the company to validate?
The focus behind this question is the fact that both EMA and FDA expect software that could interact with the yet-to-be validated quality system, and this would comprise of the learning management system (LMS) that stores records related to qualification and training.
When a worker takes part in development and performance management activities, these should not affect the qualification of the said employee, and should fall beyond the validated condition.
This article describes a model through which FDA-regulated organizations can confirm the LMS part, instead of the whole TMS, which could contain tools focusing on competency management, performance management, and goal setting.
The QA and IT validation teams as well as the TMS sponsors, which often include the HR team, can realize several benefits by directing the validation effort on the “qualification record.”
Addressing FDA validation requirements
According to FDA, “validation” is defined as the establishment of “documented evidence that provides a high degree of assurance that a specific process will consistently produce a product meeting its predetermined specifications and quality attributes.” As such, two categories of software are regulated by the FDA:
- Software that is a medical device (or a medical device accessory or component)
- Software that is employed for or to support regulated activities (such as regulatory recordkeeping manufacturing, clinical data management, and quality assurance)
FDA states that validation of computer systems is a documented, formalized procedure used for testing computer systems and software needed by Federal Regulations (21 CFR 11.10.a).
The Production and Process Controls regulations [21 CFR 820.70] include the validation requirements for medical device companies which state: “When computers or automated data processing systems are used as part of production or the quality system, the manufacturer shall validate computer software for its intended use according to an established protocol. All software changes shall be validated before approval and issuance. These validation activities and results shall be documented.”
In order to conform to FDA regulations, all the computer systems, software, spreadsheets, and databases that affect quality systems have to be validated by Life Science companies. Proper documentation for all phases of the software development life cycle (SDLC) should also be developed.
In the EU, Annex 11, a similar focus is placed on the life cycle of a product, with more emphasis also being placed on management accountability and “people” than the US regulations.
Validation activities are relevant to all applications needs such as processing requirements, inputs to be captured, outputs from the system, security, as well as business requirements.
A software vendor will be typically audited by a company to assess the vendor’s requirement traceability matrix, functional needs, business needs, system design, test plan, test scripts, and validation summary report.
For any scope of improvements, the software vendor would also be expected to send a preview period and a list of regression test scripts so that the company can assess any improvement made to the software, and also assist with the internal validation effort of that company.
21 CFR Part 11 is one key area that defines the rules for use and acceptability of electronic signatures and records in place of “handwritten” signatures and “paper” records.
Part 11 applies to any record where a company produces, maintains, modifies, archives, transmits, or retrieves in electronic form. It also applies to any record that is submitted to the FDA in electronic form. The following are the main validation requirements:
- Code and password security
- Electronic signature security
- Code and password maintenance
- Audit trails: The system should be able to store critical data inputs, time and date stamp, and also the person who did the modifications
- System security: Only authorized users who are qualified by documented training and approval should perform system administration
In order to deal with these regulatory obligations, Life Science companies carry out several activities for systems that affect the “quality system”, producing the design document, conducting performance testing, developing test scripts, developing functional and user needs, etc.
The validation effort is quantified in sunk costs, such as the time required by salaried validation and IT teams as well as adding many months to deploy the software.
Depending on the interaction with the quality system, Life Science companies would want to know which TMS tools should be validated for a comprehensive TMS that covers the requirements of quality assurance, human resources, individual departments, and leadership and development.
Most of the existing TMS can meet a host of learning and performance requirements and they come with a set of tools that focus on learning, skills development, performance management, goals management, and succession management.
Segmenting qualification from performance and development programs
A “fine line” exists between the “Skills Development Rating” and the “Qualification/ Training Record” for an employee which could be seen by certain validation teams. Talent management tools offer skill gap analysis.
Operational or Manufacturing Excellence teams with employee development programs could be assisted by such tools, but given that the “learning system” within the TMS is the qualification record, this system has to be validated based on FDA needs.
Skills development activities that merely exist to improve the performance of employees outside the baseline qualification should exist in a non-validated TMS. Several aspects of a “Talent Management Suite” can be exploited by HR teams to develop performance management programs for employees, and leadership programs for managers in the manufacturing production or operations departments.
In order to address this requirement, Life Science companies should extend development opportunities to all employees and help them develop beyond their present qualification training, without affecting their current qualifications that should be kept in an auditable format for investigators.
Let us consider a line operator working within a pharmaceutical manufacturing setting. This worker has to complete a number of curricula corresponding to his or her job function.
Captured in the LMS, these activities should be recorded in a validated system, but the company may think otherwise and decide that development programs created to measure the overall performance or goals of this employee do not have to be stored in this validated system.
During a typical GMP on-site inspection, an FDA investigator may request to assess the qualifications of an employee and the auditing team would have to present the history and documentation of qualification.
To accommodate this request, any job function training and qualification training would have to exist in the platform component that meets 21 CFR Part 11 requirements, whereas development and performance management activities would exist in a non-validated environment.
A model for segmenting compliance and talent management applications
In order to simplify the validation process, IT and QA experts at UL EduNeering have proposed a new model, where a Talent Management Suite of applications are segmented between the “Performance Management Record” and the “Learning and Qualification Record” for individual employees. Two outcomes are provided by this model:
- Reduced Validation Effort: Validation of the talent management tools is no longer required, thus preventing prolonged periods of validation and application development effort required to fulfill the validation needs; this reduced effort would help deploy the TMS system much more quickly
- Audit Accuracy: Continuous qualification and compliance records are available for audits, while development activities and performance management are not mirrored on the role-based “qualification” of employees; records related to a “developmental” program would not be asked by an FDA investigator during an external audit
Life Science companies favor a “holistic” administrator and user experience for all types of talent management and learning activities instead of having several systems to deal with the numerous compliance training and talent management training activities.
Reduced administrator training; a much easier one-stop shop employee experience; and reduced subscription costs are the benefits of using a single system. The proposed model not only provides these benefits, but also covers developmental and compliance-related programs.
An employee can complete compliance-related qualification training and also participate in performance management, competency development, or skill development programs through a single interface.
The learner can sign into a single application in the UL EduNeering model, “Compliance and qualification” training is given to meet baseline qualifications, with the performance management system storing the competency ratings and “developmental” opportunities.
For sake of audit, a qualification report can be created to indicate that the employee has met his or her training needs. If divided like this, the change control and system validation must operate in a clear, defined way so that developmental records do not affect the validated system. Figure 1 shows the advantages of a combined system.
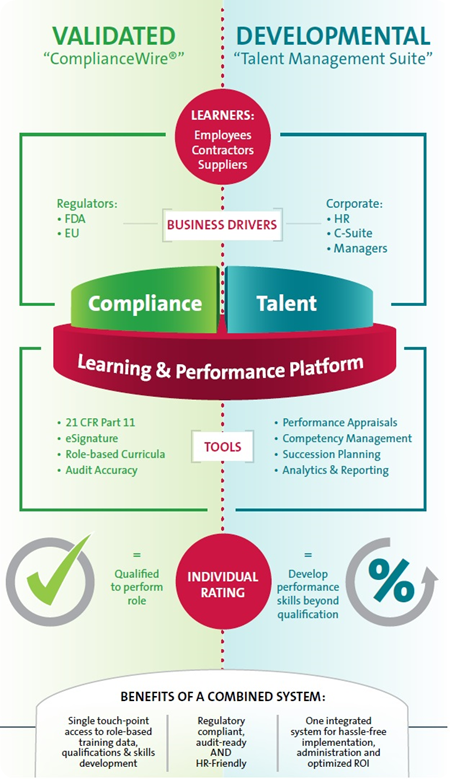
Figure 1. Benefits of a combined system
Conclusion
Life Science companies investing in talent management platforms should ensure that qualification requirements of employees comply with the stringent validation requirements stipulated by both EMA and FDA.
Department owners and HR are exploring ways to provide developmental opportunities to their employees. These programs can help with overall performance improvements and talent retention.
Manufacturing/operations benefits:
- Reduces downtime
- Enhances operational efficiency
- Provides understanding about employee skills for resource planning
- Helps employees grasp the required skills for future positions
- Allows for more effective skills transfer across employees
- Enhances production output and other KPIs
HR benefits:
- Complements all “universal competency” programs in place
- Enhances accuracy of recruitment activities as they correspond to technical skill positions
- Enhances employee retention metrics, as employees are motivated by development plans to progress to new skill levels
QA/compliance benefits:
- Allows for more efficient training
- Reduces noncompliance risks
- Aligns training activities to critical-to-quality initiatives
- Helps describe training effectiveness to external and internal auditors
With the aid of the UL EduNeering model, Life Science companies can get a single solution that addresses these benefits, while simultaneously reducing the validation effort by dividing an employee’s “developmental” record database from the “qualification” database that represents a part of the quality system.
Acknowledgements
Produced from material originally authored by Rob Sims, Life Science Practice Leader UL Compliance to Performance.
About UL Compliance to Performance
UL Compliance to Performance provides knowledge and expertise that empowers Life Sciences organizations globally to accelerate growth and move from compliance to performance.
Our solutions help companies enter new markets, manage compliance, optimize quality and elevate performance by supporting processes at every stage of a company’s evolution.
UL provides a powerful combination of advisory solutions with a strong modular SaaS backbone that features ComplianceWire®, our award-winning learning and performance platform.
UL is a premier global independent safety science company that has championed progress for 120 years. It’s more than 12,000 professionals are guided by the UL mission to promote safe working and living environments for all people.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.